Sontheimer Lab
Role of Glial in Neurological Illnesses and Cancer
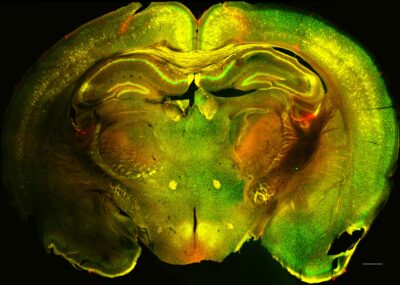 Glial cells support normal brain function by maintaining ion and neurotransmitter homeostasis, by regulating local blood flow and by stabilizing the blood brain barrier. Many diseases present with an impairment of one or multiple of these functions.
Glial cells support normal brain function by maintaining ion and neurotransmitter homeostasis, by regulating local blood flow and by stabilizing the blood brain barrier. Many diseases present with an impairment of one or multiple of these functions.
The laboratory uses multi-photon laser scanning microscopy to study glial-vascular interactions and changes thereof in diseases ranging from Alzheimer and Epilepsy to Cancer. We use electrophysiology to study cell excitability and EEG recording to study seizures.
Changes in glial function in Alzheimer Disease
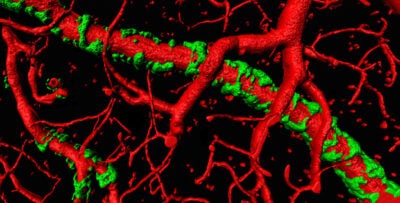
vessels with amyloid plaque attached
We are studying the hypothesis that the gradual buildup of amyloid is toxic to astrocyte process causing the retraction of their endfeet from blood vessels. This in turn causes a failure of tight junction proteins to form and the blood brain barrier breaks down causing entry of harmful blood born molecules into the brain. Loss of astrocytic endfeet also impairs activity dependent regulation of local blood flow and, the resulting hypoperfusion may underly cognitive loss in Alzheimer.
Glia as drivers of abnormal neural activity in Epilepsy
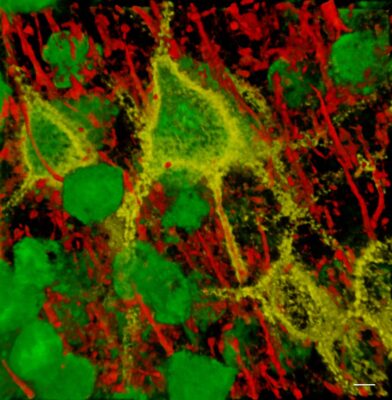
perineuronal nets, attached
Lesional epilepsy resulting from trauma, infection, tumors or stroke present with a reactive glial response called reactive gliosis. This is characterized by the release of extracellular matrix degrading proteases which alter the perineuronal matrix that stabilizes the function of GABAergic interneurons. These change their electrical properties to become hyperexcitable. Reactive astrocytes also loose their ability to clear neuronally released glutamate from the brain. Together these defects cause abnormal hyperexcitability that gives rise to seizures.
Neurodegenerative properties of malignant glial derived brain tumors
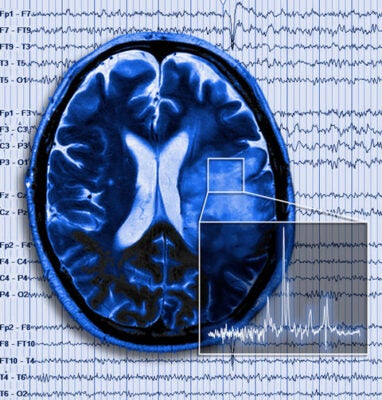
Brain tumor in patient with seizure superimposed
Gliomas are tumors derived from astrocytes or glia precursor cells. They form rapidly expanding masses that send of individuals cells to infiltrate the surrounding brain and develop satellite tumors. This diffuse infiltration makes complete surgical resection essentially impossible. Cells invade along blood vessels dislodging astrocyte endfeet from the blood vessels resulting in loss of blood brain barrier function permitting entry of blood born molecules and peripheral immune cells. The tumor-infiltrated brain becomes progressively compromised, with pronounced neuronal cell death and edema being common. This is in large parts due to assiduous release of glutamate from the tumor via a highly expressed cysteine-glutamate antiporter. This amino-acid transporter serves to supply cystine for the production of the cellular reducing agent glutathione. Hence peritumoral glutamate toxicity is a collateral damage of the tumors intrinsic synthetic activity. The underlying transporter is regulated by p53 and CD44 providing novel targets to inhibit the glutamate release underlying the neurodegenerative biology of these tumors. A pharmacological blocker was used successfully by the Sontheimer lab in a recent clinical study.
