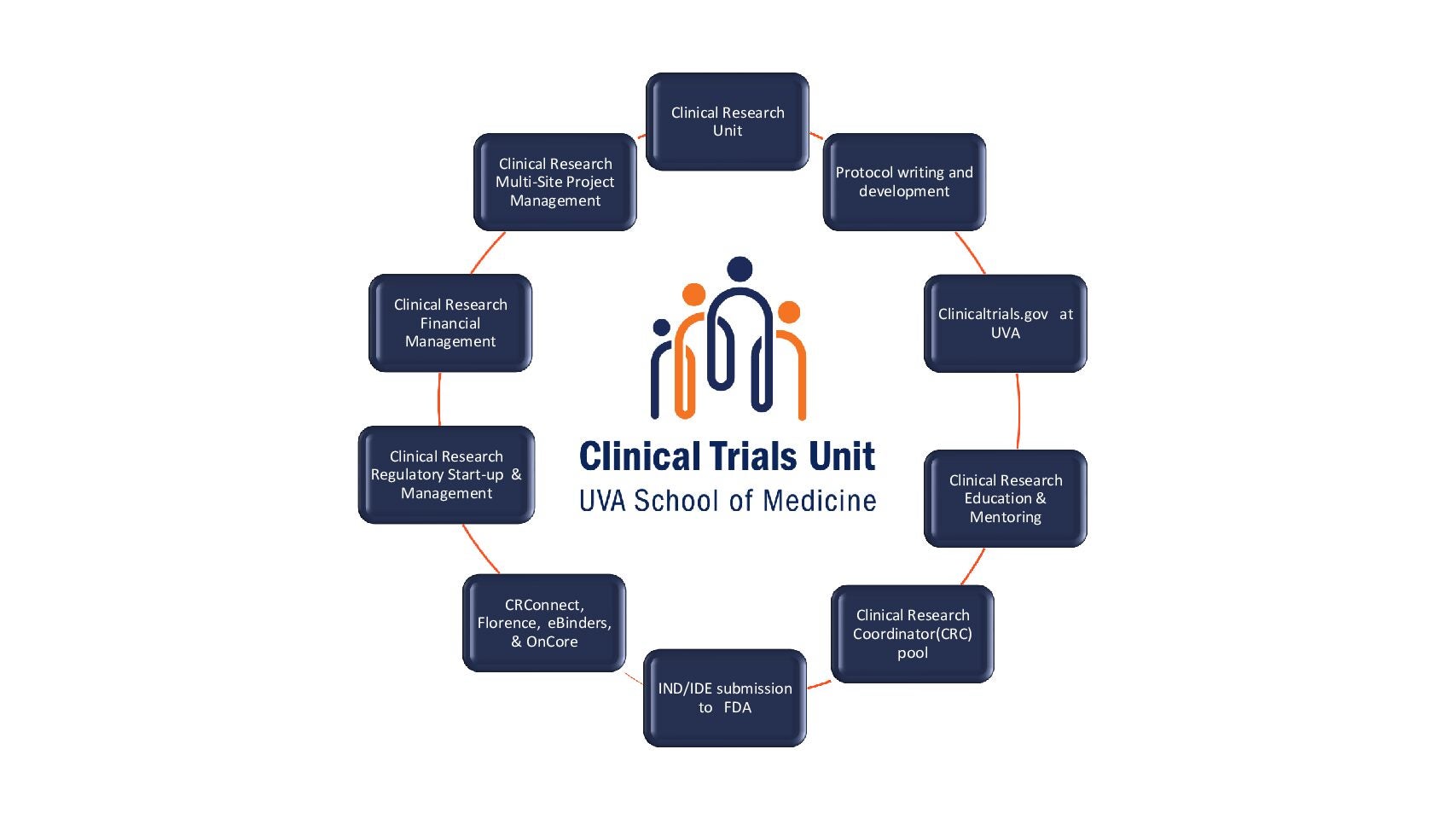
Clinical Trials Unit
The School of Medicine (SOM) has developed a new, full-service Clinical Trials Unit (CTU), intended to serve the School of Medicine, UVA Health, and the entire University of Virginia. The CTU provides a centralized infrastructure to support our clinical research in a methodologically sound, compliant, efficient, and cost-effective manner.
WHY Our goal is to assist and strengthen clinical researchers as they facilitate and execute high-quality human subject research. The CTU aids the School of Medicine, UVA Health, and the University of Virginia to achieve our shared mission to transform research and inspire hope for all Virginians and beyond.
WHO CTU leadership is comprised of Dr. Linda Duska, MD, Associate Dean for Clinical Research and Lori Elder, RN, Director of the CTU.
WHAT The CTU provides the following services:
- Clinical Research Coordinator (CRC) Pool is comprised of trained research coordinators who are experienced with all UVA systems and are for hire at an hourly rate.
- Clinical Research Education & Mentoring provides educational courses, training and one-on-one support to clinical research coordinators across UVA.
- Clinical Research Financial Management services include budget development, billing coverage analysis (BCA) development, Epic research billing assistance, and sponsor invoicing and reconciliation.
- Clinical Research Multisite Project Management is available for investigator-initiated or industry sponsored studies that involve data collection from non-UVA sites.
- Clinical Research Regulatory Start-Up & Management can assist with protocol development, IND/IDE applications, IRB submissions and the maintenance of electronic master files in Florence eBinders.
- Clinical Research Unit is an outpatient clinical research space located on grounds in the Collins Wing (with an additional unmanned location at Fontaine Research Park) available to clinical research teams for the conduct of study visits.
- ClinicalTrials.gov at UVA assists research teams with the mandatory trial registration process, ongoing record maintenance and final results submissions.
- CRConnect, Florence eBinders, OnCore is a team of professionals available as a resource to all study teams conducting clinical research utilizing these core systems at UVA.