Research
The Le Laboratory
The Le Laboratory is composed of a core group of talented research scientists, postdoctoral fellows, students and research associates. Our work bridges the fields of Cancer Biology, Developmental Biology, and Stem Cell Biology that focuses on neural-crest derived tissue development, regeneration, and tumorigenesis, in particular, the biology of neurofibromatosis, a group of genetic disorders that causes tumors to form on nerve tissue.
(Watch Dr. Le’s “Research in Motion” video here.)
Our broad research goals are to:
- Decipher mechanisms that initiate and drive human cancer
- Generate robust in vivo and in vitro models to delineate tumor progression
- Develop novel therapeutic targets for human cancers. A related goal is to translate our basic scientific discoveries in the laboratory into human clinical trials.
Our primary scientific interests include identifying the cell of origin of tumorigenesis and elucidating the roles of tumor microenvironment in cancer development. Our laboratory dissects these cellular and molecular mechanisms of tumorigenesis from the developmental perspective. We utilize Neurofibromatosis Type 1, a common tumor predisposition human genetic disorder, as a model to address these two fundamental questions in Cancer Biology as well as elucidating cutaneous nervous system development and regeneration.
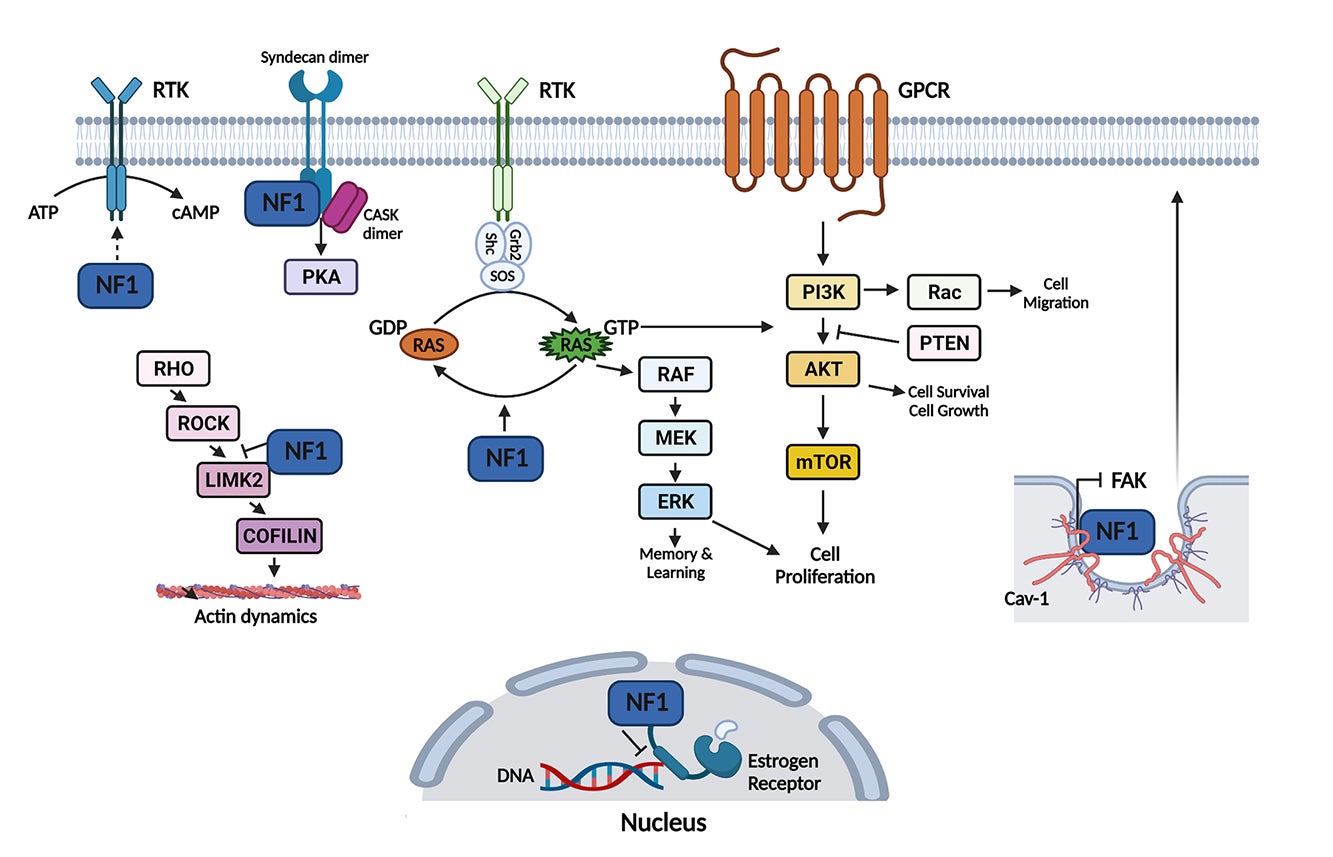
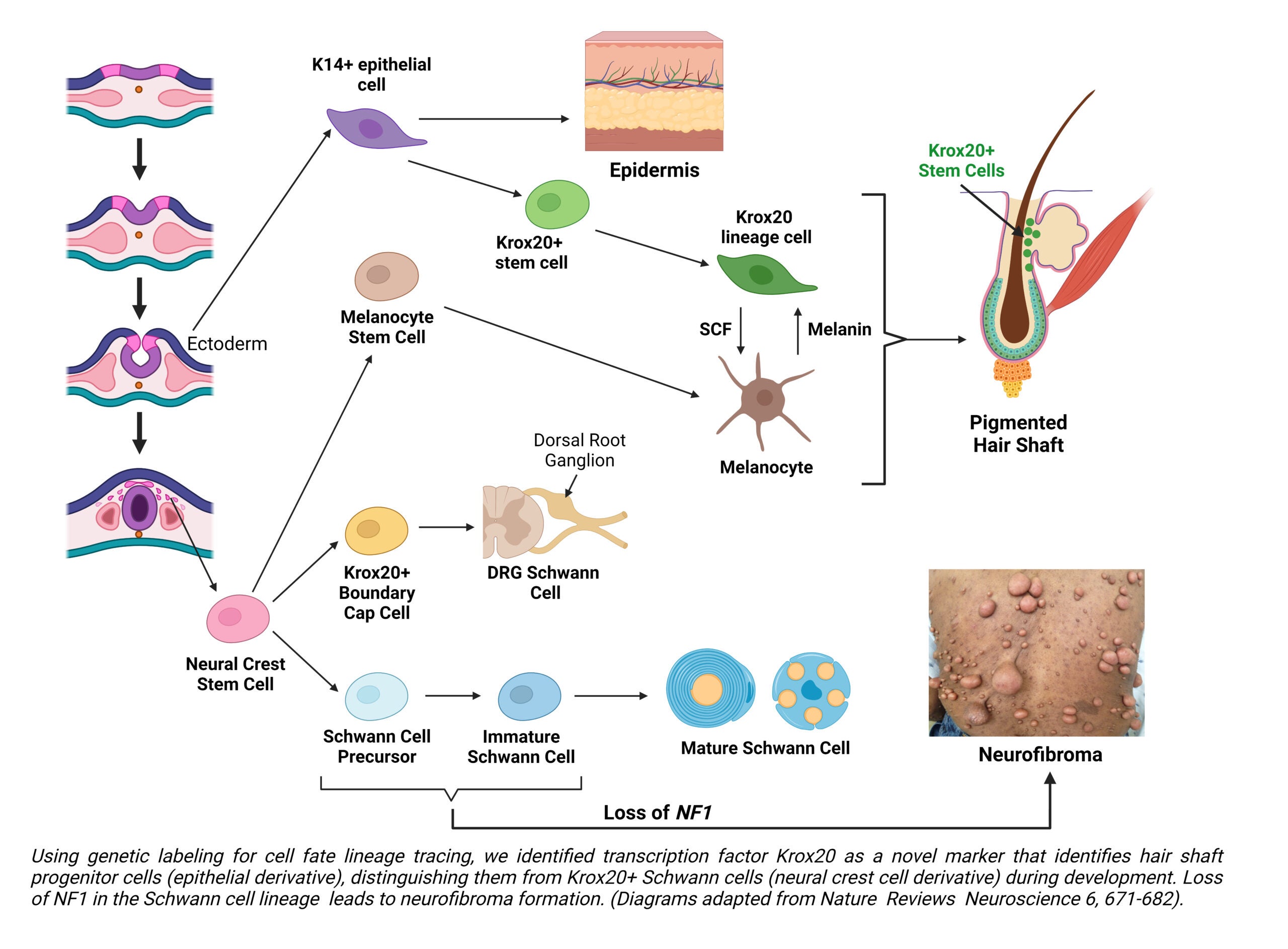
A major contribution of our laboratory is the generation and exploitation of novel neurofibromatosis models to elucidate mechanisms that initiate development of neurofibroma, a Schwann cell tumor, and drive their malignant transformation. Our work has identified the cells of origin for different types of neurofibroma [Cancer Cell, 26(5):695-706; Cell Stem Cell, 4(5):453-463], defined the developmental “window-of-opportunity” within the Schwann cell lineage for neurofibroma development [Cancer Research, 71(13):4686-4695], and delineated vital cancer pathways for its malignant transformation into Malignant Peripheral Nerve Sheath Tumors, which account for 10% of all soft tissue sarcomas, and are lethal cancers whose pathways of activation are poorly understood [Cell, 152(5): 1077-1090; Cell Reports, 6(1):81-92; Cancer Research, 74(2):586-597]. These and current studies in our laboratory address fundamental unanswered questions in the neurofibromatosis field. They will not only provide important insights into the molecular and cellular pathogenesis of neurofibromatosis, but could also lead directly to novel and potentially effective therapies aimed at delaying or preventing tumor formation in neurofibromatosis patients, where no curative treatment exists today.
In addition, it was this work jn neurofibromatosis and mechanisms whereby neural crest-derived tissues and nerves can affect tumor development in skin and other tissues that serendipitously led us to uncover the identity of follicular epithelial cells that directly give rise to hair and the mechanisms that cause hair to turn gray [Genes & Development, 31(8): 744-756] – findings that could one day help identify possible treatments for balding and hair graying.
Neurofibromatosis
Neurofibromatosis encompasses a group of three complex neuro-cutaneous genetic disorders that cause tumors to form on nerve tissues and other manifestations. They arise from changes in different genes and lead to different clinical presentations. The three distinct types of neurofibromatosis are:
