Research
Seizure-Induced Apnea Initiates Death in Mouse Models of SUDEP
In previous work, we used the combined recording of EEG, ECG, and breathing (Fig. 1) to observe that seizure-induced apnea occurred during the tonic phase of seizures from patients with SCN8A epileptic encephalopathy are nearly identical to those observed during spontaneous seizures in Scn8a mutant mouse models (Wenker et al., 2021, Annals of Neurology). In the mice, seizures proved fatal when breathing did not resume at the end of the tonic phase, indicating breathing, but not cardiac, dysfunction is the primary cause of seizure-induced death. Since then, my laboratory has made a number of important additional discoveries relating to the mechanisms of seizure-induced apnea and SUDEP:
1) We demonstrated that the large changes in cardiac rate were due to fluctuations in autonomic activity and, importantly, exogenous suppression of cardiac activity was not sufficient to produce seizure-induced death (Wenker et al., 2022, Frontiers in Neuroscience)
2) Functional adrenergic signaling is critical for breathing recovery after tonic seizures (Wengert et al., 2021, Frontiers in Neuroscience)
3) Suppression of cortical activity, including the motor cortex, does not affect the tonic phase or seizure-induced apnea (Wenker et al., 2022, Frontiers in Neural Circuits), implicating brainstem neural circuitry in producing seizure-induced apnea and setting the stage for Projects 1-3, described below.
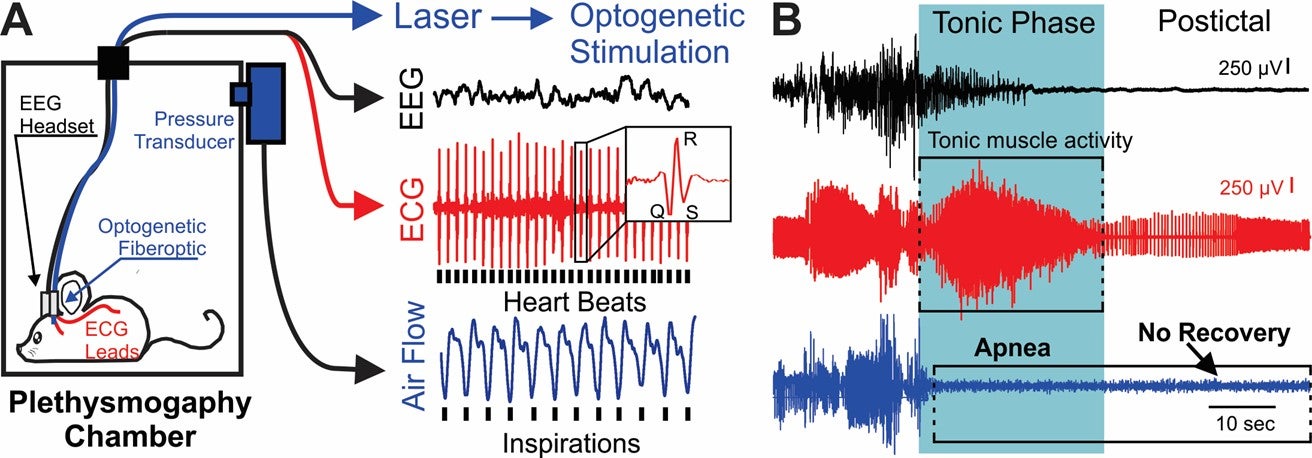
Figure 1. A) Diagram of setup. B) EEG, ECG, and breathing (pleth) recording during a spontaneous seizure-induced death (aka SUDEP).
Project 1: Neural Circuitry of Seizure-Induced Apnea and SUDEP
This project will be supported by the NIH (R01 NS133139). The primary focus of this project is to dissect the neural circuitry of seizure-induced apnea and its contribution to SUDEP. As discussed above, my laboratory has demonstrated that seizure-induced apnea contributes to seizure-induced death (aka SUDEP) in mouse models. In addition, we have shown that cortical seizure activity does not impact seizure-induced apnea1 (Wenker et al., 2022, Frontiers in Neural Circuits), nor does inhibition of the inspiratory oscillator (Fig. 2), suggesting seizure spreads through the brainstem but bypasses homeostatic elements of respiratory control circuitry. We hypothesize that increased midbrain and pontine excitability is both necessary and sufficient to produce seizure-induced apnea; the parabrachial/kölliker fuse (PB/KF) nucleus in the pons represents a key nodal point for seizure-induced apnea generation, and that brainstem nuclei, like the PB/KF and peri-aqueductal gray (PAG), are recruited specifically during seizure-induced apnea.
![Figure 2. [All light blue shading represents photostimulation of 20 ms pulses at 20 Hz.] A, BötC photostimulation inhibits inspiratory activity. B-C, Audiogenic seizures in D/+ mice with no photostimulation (B) or 1 s, 2 Hz, trains during apnea. D, Apnea duration was unchanged by BötC photostimulation protocols designed to mimic slow breathing (p = 0.7162; 1-way ANOVA).](https://med.virginia.edu/wenker-lab/wp-content/uploads/sites/474/2023/06/Fig2-002.png)
Figure 2. [All light blue shading represents photostimulation of 20 ms pulses at 20 Hz.] A) BötC photostimulation inhibits inspiratory activity. B-C) Audiogenic seizures in D/+ mice with no photostimulation (B) or 1 s, 2 Hz, trains during apnea. D) Apnea duration was unchanged by BötC photostimulation protocols designed to mimic slow breathing (p = 0.7162; 1-way ANOVA).
Project 2: Mechanisms of Premature Mortality in Tuberous Sclerosis Complex
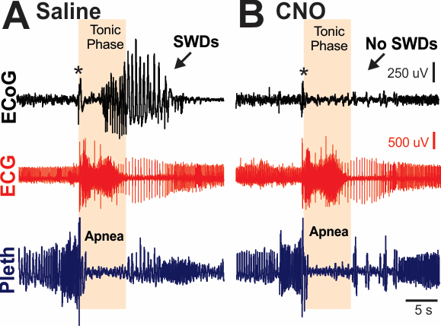
Figure 3. Cortical ictal activity is not required for seizure-induced apnea. A) Normally, a brief attenuation rapidly evolving to cortical spike wave discharge is coincident with the tonic phase and apnea. B) However, inhibition of cortical neurons with the selective ligand CNO inhibits the cortical ictal activity, including the attenuation, spike wave discharges (SWDs), and postictal EEG suppression, but had no effect on the tonic phase or apnea. Asterisks indicate movement artifact.
This project is supported by the DOD (HT94252310378). Patients with Tuberous sclerosis complex (TSC) have an extremely high risk of SUDEP, and there are commercially available TSC mouse models that die from seizures. However, there are no studies examining the mechanisms of early mortality in these models.
Considering the mechanism of epilepsy is very different in TSC compared to SCN8A epilepsy, this project stands to identify commonalities and differences in epilepsy-related fatalities. Our primary hypothesis is that apnea occurs during the tonic phase of seizures in TSC mice, and death occurs when breathing does not recover immediately postictal. Eliminating cortical seizure activity by the GABA-AT inhibitor Vigabatrin does not prevent death in TSC mouse models. Our recent data indicate that tonic seizures and the related apnea can still occur without cortical ictal activity (Fig. 3). Thus, our secondary hypothesis is that vigabatrin only stops cortical ictal activity, and hindbrain ictal activity produces apnea and death.
Project 3: Minimum Neural Circuitry of Seizures for Genetic Anti-Seizure Therapies
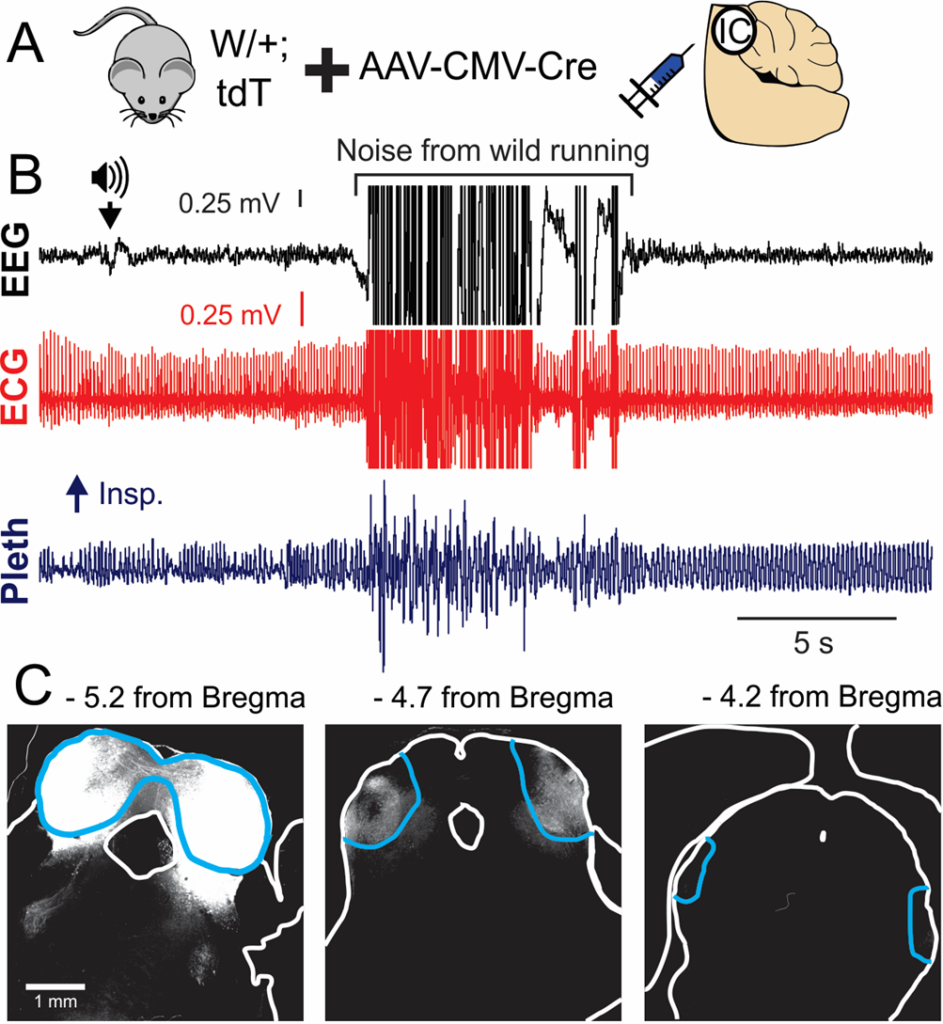
Figure 4. A) Cre expression in the inferior colliculus (IC) of W/+;LSL-tdTomato mice. B) EEG, ECG, and breathing (pleth) recording of an audiogenic seizure. C) Cre-mediated reporter expression is restricted to the IC (blue outline).
Preclinical evidence has demonstrated the effectiveness of antisense oligonucleotides (ASOs) in treating the seizure and mortality phenotype of Scn8a DEE mice. However, side effects due to brain-wide Scn8a suppression are nontrivial and include intellectual, behavioral, and motor problems. We propose to determine the minimum neural circuitry required for ASO therapy to suppress convulsive seizures, to support future targeted therapies for SCN8A and other epilepsy patients.
We have produced data showing that expression of an Scn8a mutant allele selectively in the inferior colliculus (IC) is sufficient to produce convulsive audiogenic seizures (Fig. 4). We hypothesize that selective inhibition of the inferior colliculus (IC) with ASOs is sufficient to blunt or eliminate seizures and increase survival in Scn8a mutant mice. The goals of this project include determining whether ASO delivery into the IC can 1) inhibit audiogenic seizures and 2) increase survival.
