About
The Center for Human Therapeutics (CHT) cGMP Facility
We offer manufacturing of cellular and biologics for safety and initial efficacy testing in first–in–
human, pilot, and Phase I clinical trials. Our mission is to facilitate bench–to–bed translation into
novel cellular therapies. We provide focused scientific, technical, and regulatory support for
investigator-initiated investigational new drug applications (INDs) in cell and gene therapy.
Current and completed trials include hematologic malignancies multiple myeloma, breast cancer,
prostate cancer, glioblastoma, neuroblastoma, and pancreatic cancer. The potential applications
of cellular therapy and biologics are not limited to cancer, a large number of disease indications
such as cardiovascular, autoimmune diseases, diabetes, neurological diseases, etc.
The Center for Human Therapeutics (CHT) facilitates bench–to–bedside translational of preclinical
biologics at state of a art Good Manufacturing Practices (cGMP) compliant facility at the
University of Virginia (UVA). The facility is designed in accordance with U.S. Food and Drug
Administration (FDA) as well as other relevant requirements for controlled clean room
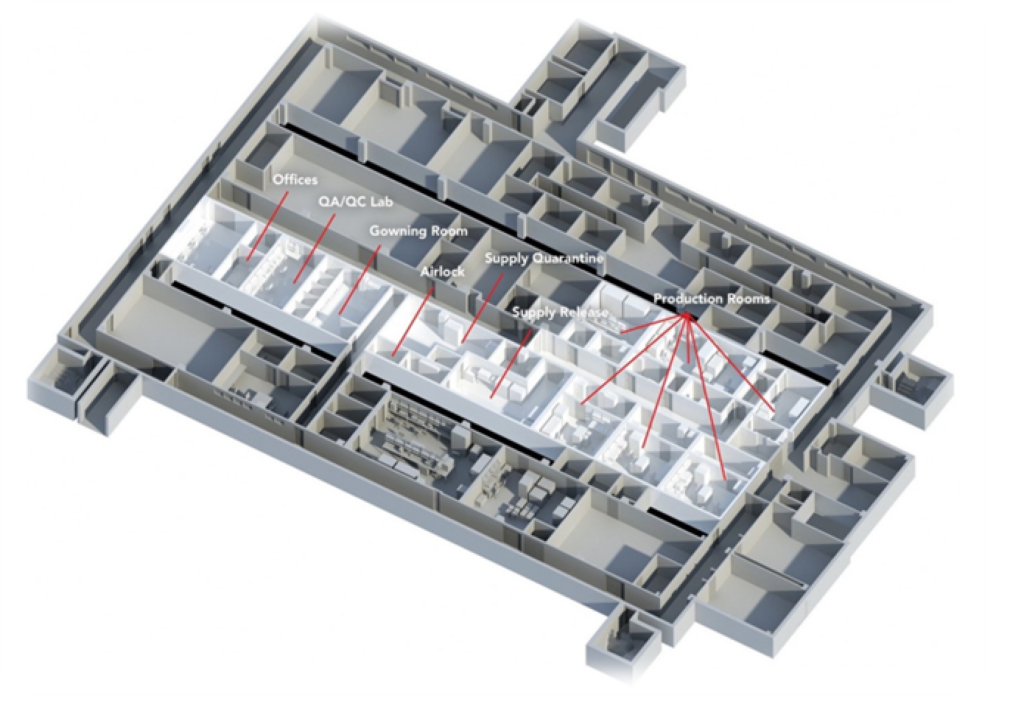
manufacturing environments and aseptic processing. The facility includes 6 manufacturing rooms
(All six rooms are ISO 7) controlled access corridor, office, and support space. The CHT cGMP is a
dedicated multi–use clean room complex for the manufacturing and processing of cellular
products, preparation of islet cells for transplantation, production of monoclonal and bispecific
antibodies, manufacturing of viral vectors, and production of solution-phase nanoparticles for
human clinical use.
Target Cliental
Our target cliental are BIOLOGICS translational researchers within UVA as well as outside the
UVA investigators who want to start phase I/II clinical trial under new or existing IND. Support
for Clinical Grade Biologics Include:
- Cell therapy products
- Monoclonal antibodies
- Bispecific antibodies
- Viral vector preparation
- Vaccine production/vaccine vialing