Maria Luisa S. Sequeira-Lopez, MD
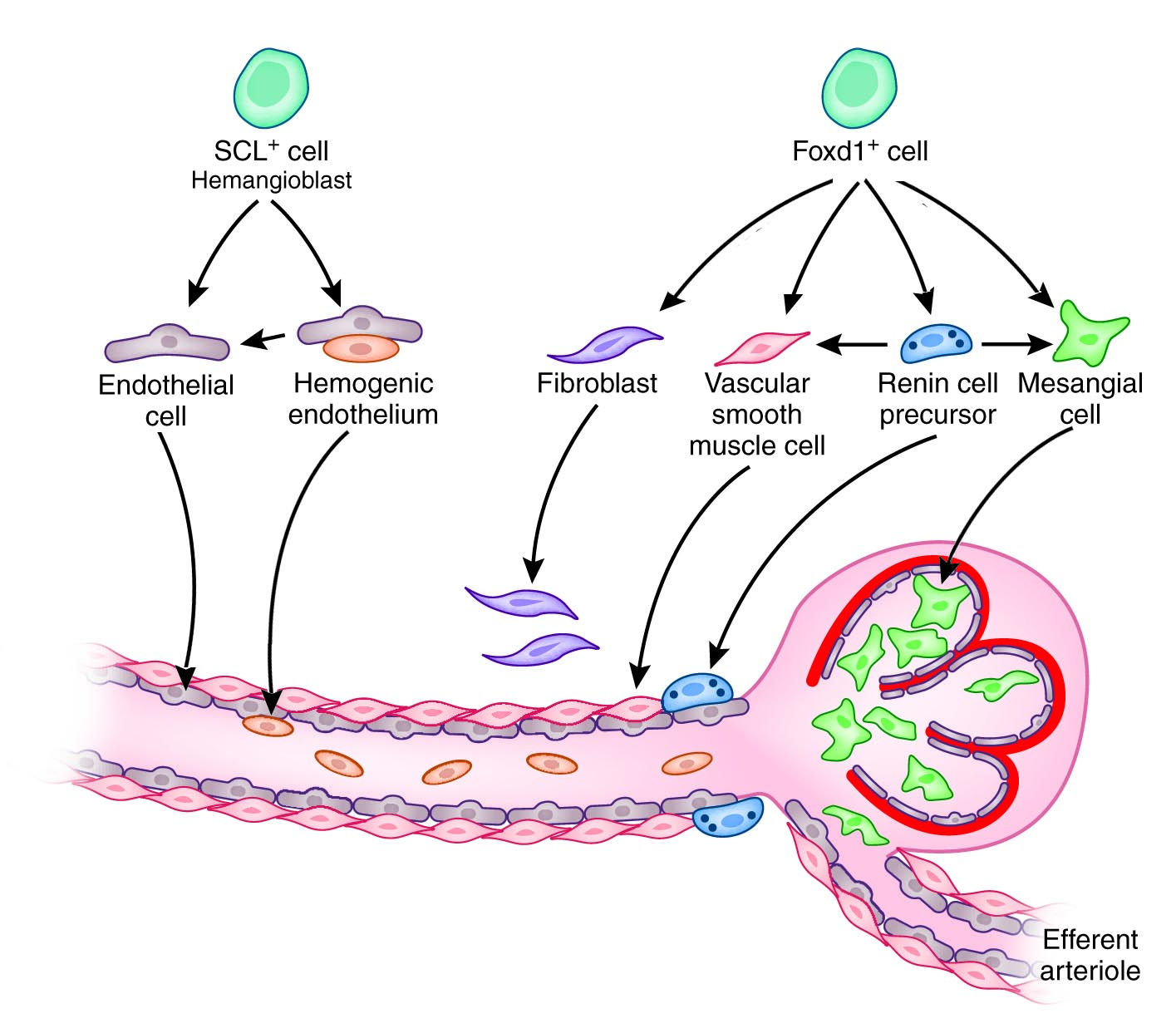
 A main goal of our research program is to define the precise cellular origin and mechanisms whereby renal precursor cells lead to the successful formation of the renal arterial tree, without which there is no functioning kidney. We hypothesize that the embryonic renal stromal compartment has at least two distinct early progenitor cells that give rise to all other cells of the kidney arterioles and their perivascular compartment: 1) A precursor of hemogenic endothelium (Scl+, hemangioblast) capable of giving rise to erythroid and endothelial cells (ECs) of the renal arteriole and 2) A Foxd1+ cell from which all other vascular and perivascular/adventitial cells originate. Further, renal hemangioblasts may give rise to Flk1+ precursors that in turn may contribute not only hemogenic ECs but also vascular SMCs.
A main goal of our research program is to define the precise cellular origin and mechanisms whereby renal precursor cells lead to the successful formation of the renal arterial tree, without which there is no functioning kidney. We hypothesize that the embryonic renal stromal compartment has at least two distinct early progenitor cells that give rise to all other cells of the kidney arterioles and their perivascular compartment: 1) A precursor of hemogenic endothelium (Scl+, hemangioblast) capable of giving rise to erythroid and endothelial cells (ECs) of the renal arteriole and 2) A Foxd1+ cell from which all other vascular and perivascular/adventitial cells originate. Further, renal hemangioblasts may give rise to Flk1+ precursors that in turn may contribute not only hemogenic ECs but also vascular SMCs.
The lineage relationship among all these cell types has not been clarified and the mechanisms underlying the differentiation and assembly of the kidney arterioles are unclear. Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid metabolite crucial in many biological processes, including angiogenesis. S1P1 and S1P3 are highly expressed in precursors and definitive cells of the kidney arteriole and may play an important role in the differentiation and assembly of arteriolar cells. We are currently testing two interrelated hypotheses: 1) The renal arterial tree originates from hemogenic and non-hemogenic- stromal cell precursors 2) Locally generated S1P (by hemogenic endothelium) interacting with S1P1 and S1P3 receptors is crucial for the maturation and assembly of the renal arterioles. There is currently very limited information regarding the crucial events that govern the morphogenesis of the renal arterial tree. This work aims to solve an existing challenge and generate new and exciting information of relevance to the fields of regeneration and hemo-vascular development with the potential to benefit children and adults with kidney and vascular diseases.
Obstructive nephropathy is the leading identifiable cause of renal failure in children. However, current management is limited to surgical release of obstruction, which often fails to prevent progression. Our research program aims to investigate the underlying renal cellular mechanisms, using a model of reversible variable chronic partial unilateral ureteral obstruction (UUO) in the neonatal mouse, which parallels urinary tract obstruction in the human fetus. Rather than the existing paradigm of using renal interstitial fibrosis as an experimental end-point, we are examining the injury to the glomerulotubular junction. Death of epithelial cells in this nephron segment is an important but under-recognized pathway that leads to the formation of atubular glomeruli (ATG) and aglomerular tubules. Persistent UUO in the neonatal mouse leads to widespread formation of ATG, and early release of the obstruction stops this process, allowing significant remodeling of the renal parenchyma. We are currently trying to understand the role of the Notch signaling pathway (receptors and ligands) in the generation of ATG and in the remodeling/regeneration process after release of obstruction. Elucidation of mechanisms of tubular injury, rather than of extracellular matrix deposition may lead to the development of new therapies to allow slowing or reversal of progression that occurs prior to the development of interstitial fibrosis.
Additional major projects performed in collaboration with Dr. Gomez and Dr. Belyea include:
- Defining the origin, fate and function of renin precursors in extra renal tissues.
- Understanding the role of renin precursors in normal and neoplastic hematopoiesis.
Contact Information:
Phone: 434-924-5065
Email: msl7u@virginia.edu