SCN8A Epileptic Encephalopathy
Overview
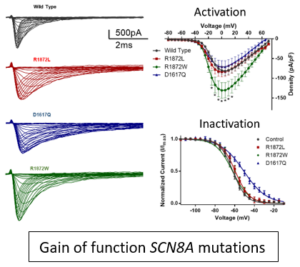 Pediatric epilepsy is a common neurological disorder, affecting between 300 and 600 patients per 100,000, with up to 30% of these cases being considered intractable. Many de novo missense mutations of SCN8A, the gene that encodes for Nav1.6, have been identified and patients have seizure onset between birth and 12 months of age. Our lab has begun to evaluate a number of these mutations by expressing the mutations in cell lines and recording sodium channel currents using patch clamp electrophysiology techniques. Our studies have shown that Nav1.6 mutations associated with epilepsy are gain-of-function (GOF) mutations while Nav1.6 mutations associated with intellectual disability (ID) are loss-of-function (LOF) mutations.
Pediatric epilepsy is a common neurological disorder, affecting between 300 and 600 patients per 100,000, with up to 30% of these cases being considered intractable. Many de novo missense mutations of SCN8A, the gene that encodes for Nav1.6, have been identified and patients have seizure onset between birth and 12 months of age. Our lab has begun to evaluate a number of these mutations by expressing the mutations in cell lines and recording sodium channel currents using patch clamp electrophysiology techniques. Our studies have shown that Nav1.6 mutations associated with epilepsy are gain-of-function (GOF) mutations while Nav1.6 mutations associated with intellectual disability (ID) are loss-of-function (LOF) mutations.
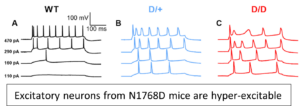 In collaboration with Dr. Miriam Meisler at the University of Michigan, we have begun investigating how disruptions in Nav1.6 channel activity, as a result of a site specific mutation, can lead to increases in neuronal activity and seizure onset using two human knock-in mouse models of SCN8A epileptic encephalopathy (N1768D (D/+) and R1872W (W/+)).
In collaboration with Dr. Miriam Meisler at the University of Michigan, we have begun investigating how disruptions in Nav1.6 channel activity, as a result of a site specific mutation, can lead to increases in neuronal activity and seizure onset using two human knock-in mouse models of SCN8A epileptic encephalopathy (N1768D (D/+) and R1872W (W/+)). 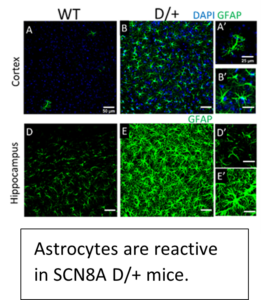 Our current studies have focused on the role of excitatory neurons (mEC and layer V/VI somatosensory cortex) and inhibitory neurons (PV, SST and VIP) in the development of seizures using these clinically relevant mouse models. More recently, we have begun to determine a role for microglia and astrocytes in the development of seizures in SCN8A epileptic encephalopathy. Since the R1872W mouse model is a Cre-dependent knock in mouse model, it allows specific expression of the GOF mutation within specific cell types and selective regions of the brain.
Our current studies have focused on the role of excitatory neurons (mEC and layer V/VI somatosensory cortex) and inhibitory neurons (PV, SST and VIP) in the development of seizures using these clinically relevant mouse models. More recently, we have begun to determine a role for microglia and astrocytes in the development of seizures in SCN8A epileptic encephalopathy. Since the R1872W mouse model is a Cre-dependent knock in mouse model, it allows specific expression of the GOF mutation within specific cell types and selective regions of the brain.
Ongoing and Future Projects
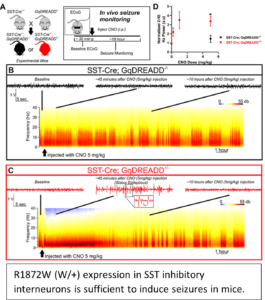 Our current ongoing studies are focused on understanding the role of inhibitory interneurons in seizure initiation in SCN8A epileptic encephalopathy mice. Using our R1872W Cre-dependent mouse model we will determine the effects of specific expression of R1872W in PV interneurons (Scn8a-PVW/+). Furthermore, we will determine if GqDREADD-mediated chemogenetic activation of PV interneurons is sufficient to either suppress seizures or initiate seizures. We will investigate if silencing of Nav1.6 using virally delivered shRNA will attenuate proexcitatory Na channel activity, “normalizing” neuronal excitability in both excitatory and inhibitory neurons and reduce seizure activity in SCN8A epileptic encephalopathy mice. These experiments will establish the importance of targeting either excitatory or inhibitory neurons in a model of human epileptic encephalopathy.
Our current ongoing studies are focused on understanding the role of inhibitory interneurons in seizure initiation in SCN8A epileptic encephalopathy mice. Using our R1872W Cre-dependent mouse model we will determine the effects of specific expression of R1872W in PV interneurons (Scn8a-PVW/+). Furthermore, we will determine if GqDREADD-mediated chemogenetic activation of PV interneurons is sufficient to either suppress seizures or initiate seizures. We will investigate if silencing of Nav1.6 using virally delivered shRNA will attenuate proexcitatory Na channel activity, “normalizing” neuronal excitability in both excitatory and inhibitory neurons and reduce seizure activity in SCN8A epileptic encephalopathy mice. These experiments will establish the importance of targeting either excitatory or inhibitory neurons in a model of human epileptic encephalopathy.
