Fernando Ruiz-Perez, PhD
My research interest focuses on understanding the role of virulence factors implicated in the pathogenesis of diarrheagenic E.coli, which primarily affects children.
The mortality and morbidity associated with enteric infections remain a significant public health problem. The World Health Organization (WHO) ranks diarrheal disease as the second most common cause of mortality among children under the age of 5. In the United States, the Center for Disease Control and Prevention (CDC) has estimated that more than 200 million episodes of acute gastroenteritis occur annually, resulting in nearly 1 million hospitalizations and over 5000 deceases.
Recent data from the Global Enteric Multi-Center Study (GEMS), one of the largest case-control studies aiming to understand the burden of pediatric diarrheal disease revealed that pathogenic E. coli and Shigella spp are among two of the four main causative agents of moderate to severe diarrhea among children in endemic areas, yet vaccines for these agents are not available. An obstacle to such vaccine efforts is the limited knowledge on the repertoire of virulence mechanisms that enteropathogens have evolved to colonize, multiply and escape host defenses. Therefore, understanding the mechanisms of how such sophisticated virulence factors impact the immune system during enteric infections is a research priority. In this rubric, my research focuses primarily in elucidating the role in pathogenesis of an expanding family of virulence factors denominated “Serine Protease Autotransporters from Enterobacteriacea” (SPATE), whose members resemble those belonging to the trypsin-like super-family of serine proteases.
SPATE proteins are produced by enteric pathogenic E. coli, Shigella and recently, also found in Salmonella, Edwardsiella, and Citrobacter species(2). Furthermore, SPATEs have been found not only in all recognized E. coli pathotypes; agents of enteric/diarrheal disease, but also in extraintestinal E. coli pathogens [ExPEC], such as uropathogenic E. coli [UPEC] and septicemic E. coli, which are responsible for urinary tract infections [UTIs] and neonatal sepsis/meningitis, respectively.
We recently showed that a subfamily of SPATE proteins have lectin-like properties and exhibit protease activity to a remarkable array of glycoproteins and which are substituted with carbohydrates structurally similar to those found on human mucin glycoproteins(1,4). Most significantly, cleavage of glycoproteins by SPATEs triggered a number of effects on human leukocyte functions in vitro, including apoptosis, cell activation, and defects in chemotaxis and cell migration(4). Our studies suggest that class-2 SPATEs function as immunomodulators at several levels of the immune system. Our goal is to dissect the various mechanisms that SPATEs may exert to regulate immune functions during bacterial infections.
In addition to expanding our knowledge in the pathogenic roles of SPATEs, whose substrate specificities were largely unknown, our research program will address the potential to manipulate the SPATEs for vaccines purposes and to develop therapeutic strategies to treat numerous inflammatory disorders.
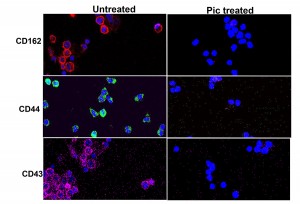
Fig 1. Effect of Class-2 SPATEs on leukocytes. Pic produced from Shigella flexneri and pathogenic E. coli degrades a broad range of leukocyte glycoproteins. To visualize degradation of O-linked glycoproteins by Pic, whole blood leukocytes were treated with Pic or left untreated for 30min, stained for DNA (blue), PSGL-1(red), CD43(pink) and CD44(green), and analyzed by confocal microscopy. Complete degradation of these glycoproteins can be observed on the Pic-treated leukocytes. Degradation of three glycoproteins are only shown, but the array of Pic targets also included CD34,CD93, CD45, CX3CL1, CD164 and Tim (T cell immunoglobulin and mucin) family.
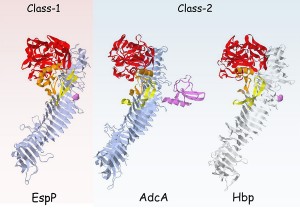 Fig 2. Stereo ribbon diagrams showing the overall structure of the entire passenger region of class 1 and class-2 SPATEs. EspP(3SZE) and Hbp(1WXR) PDB annotations were used to represent prototype structures of class-1 and class-2 SPATE proteins, respectively (adapted from reference 2). The bifurcation of SPATEs into two classes is consistent with structural differences and biological effects. Class-2 SPATEs target O-linked glycoproteins existent in nearly all cells of the hematopoietic lineage.
Fig 2. Stereo ribbon diagrams showing the overall structure of the entire passenger region of class 1 and class-2 SPATEs. EspP(3SZE) and Hbp(1WXR) PDB annotations were used to represent prototype structures of class-1 and class-2 SPATE proteins, respectively (adapted from reference 2). The bifurcation of SPATEs into two classes is consistent with structural differences and biological effects. Class-2 SPATEs target O-linked glycoproteins existent in nearly all cells of the hematopoietic lineage.
Contact Information:
Phone: 434-243-1967
Email: fr3d@virginia.edu
- Ayala-Lujan JL, Vijayakumar V, Gong M, Smith R, Santiago AE, and Ruiz-Perez F. Broad spectrum activity of a lectin-like bacterial serine protease family on human leukocytes. PloS ONE. 2014.
- Ruiz-Perez F and Nataro JP. Bacterial serine proteases secreted by the autotransporter pathway: classification, specificity, and role in virulence. Cell Mol Life Sci 2014;72:745-70.
- Vijayakumar V, Santiago A, Smith R, Smith M, Robins-Browne RM, Nataro JP, Ruiz-Perez F. Role of class-1 serine protease autotransporter in the pathogenesis of Citrobacter rodentium colitis. Infect Immun 2014;82:2626-36.
- Ruiz-Perez F, Wahid R, Faherty CS, Kolappaswamy K, et al. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc Natl Acad Sci USA 2011;108:12881-6.

