Research
N. gonorrhoeaeis
N. gonorrhoeaeis an obligate human bacterial pathogen that causes the sexually transmitted infection gonorrhea. Gonorrhea is a major public health problem because of rapid acquisition of antibiotic resistance (the CDC and WHO have identified N. gonorrhoeae as a highest priority superbug threat), the lack of protective vaccines, the fact that previously infected individuals remain susceptible to re-infection, and the predisposition of infected individuals to acquisition of HIV.
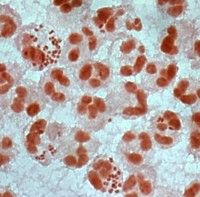
Since N. gonorrhoeae has no niche outside of humans, the biology of this organism is exquisitely tailored to life at human mucosal surfaces. Not only is N. gonorrhoeae able to exploit the nutritional and environmental conditions at these sites, the bacterium also has an extraordinary ability to thwart challenges by the host immune response. N. gonorrhoeae evades humoral immune recognition by undergoing extensive variation of its surface structures, including its type IV pili, opacity-associated (Opa) proteins, and lipooligosaccharide. N. gonorrhoeae is also highly adept at surviving confrontations with the innate immune system. Acute gonorrhea is a highly inflammatory disease characterized by the production of an exudate containing large numbers of neutrophils. Although neutrophils are the body’s first defenders against bacterial and fungal challenge, neutrophils are ineffective at killing N. gonorrhoeae, and gonorrheal exudates contain viable, infectious bacteria.
Our research focuses on how neutrophils migrate to sites of N. gonorrhoeae infection, and once there, recognize and attempt to kill the bacteria – and how the bacteria in turn resist these antimicrobial mechanisms. We apply a combination of cell biology, molecular biology, bacterial genetics, genomics, and biochemistry to address these questions. The insights gleaned will help us to understand how the mucosal innate immune system defends against infection, and how pathogens exploit mucosal defenses to enable colonization and transmission.
Our research questions include
How are neutrophils recruited during infection with N. gonorrhoeae? We have found that N. gonorrhoeae robustly infects polarized human endocervical epithelial cells and this is sufficient to drive the recruitment of neutrophils across these model epithelia. We are investigating the epithelial and bacterial factors coordinating this response and the consequence of transepithelial migration on the ability of neutrophils to respond to and combat N. gonorrhoeae.
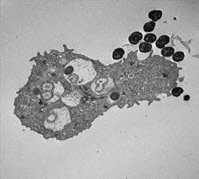
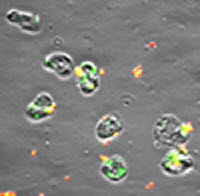
How does N. gonorrhoeae survive exposure to human neutrophils? In collaboration with the Virginia Department of Health, analysis of neutrophils from patients with acute gonorrhea suggests that the bacteria can live inside these otherwise antimicrobial cells. We developed an ex vivo assay system using adherent, interleukin 8-treated primary human neutrophils to mimic the tissue-migrated state of neutrophils in acute gonorrhea, and used it to show that N. gonorrhoeae can survive in association with them. Fluorescence-based microscopy assays for quantifying bacterial survival inside and outside of human neutrophils over time have shown that some N. gonorrhoeae are found in immature phagosomes that delay fusion with primary granules, in which the bacteria are more likely to survive. We also found that N. gonorrhoeae releases a nuclease that degrades neutrophil extracellular traps, enabling survival of extracellular bacteria. The cellular and bacterial mechanisms underlying these observations are being explored.
How does N. gonorrhoeae resist neutrophil antimicrobial activities? In collaboration with Dr. Hervé Tettelin of the Institute for Genome Sciences at the University of Maryland, we conducted dual RNA-seq analysis of N. gonorrhoeae and primary human neutrophils, alone and in co-culture. We have also conducted transposon-seq to identify genes that contribute to bacterial survival after exposure to neutrophils. Results from these studies have generated new hypotheses regarding how N. gonorrhoeae survives after exposure to neutrophils. We have identified N. gonorrhoeae gene products that defend the bacteria from neutrophils and their antimicrobial components, including efflux pumps and modifications to lipooligosaccharide. We have also identified factors derived from the human host that N. gonorrhoeae binds and exploits in order to resist phagocytic killing by neutrophils.
How do opacity-associated (Opa) proteins modulate N. gonorrhoeae interactions with human epithelial cells and neutrophils? The Opa outer-membrane proteins are a family of phase-variable bacterial adhesins. Receptors for Opa proteins include human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) and/or heparan sulfate proteoglycans, but each Opa has a unique sequence and receptor-binding capacity. We are collaborating with Dr. Linda Columbus in the UVA Department of Chemistry to explore the structure-function relationships in the Opa protein family and how changes in Opa sequence and consequent receptor binding affect bacterial interactions with human cells.
How does N. gonorrhoeae overcome nutritional immunity? Host organisms prevent invading pathogens from accessing essential metals by releasing proteins that bind these metals with high affinity. Remarkably, pathogenic Neisseria express TonB-dependent transporters that bind and extract iron and zinc from human metal-sequestering proteins like transferrin, calprotectin, and psoriasin. In collaboration with Drs. Cynthia Nau Cornelissen of Georgia State University, Walter Chazin of Vanderbilt University, and Nicholas Noinaj of Purdue University, we are investigating how these transporters are expressed and function in the context of infection to enable gonococcal survival under metal-restricted conditions.
We are more generally interested in how N. gonorrhoeae acquires nutrients during neutrophil challenge. In collaboration with Dr. Jason Papin of the UVA Department of Biomedical Engineering, we are generating a genome-scale metabolic network reconstruction of N. gonorrhoeae and are integrating it with transcriptomics and growth assays to define gonococcal growth parameters in rich media and during infection.
Considerations for designing a vaccine against N. gonorrhoeae? A decades-long goal of public health has been the development of a gonorrhea vaccine. The Criss lab is examining how antibodies elicited by preclinical vaccine candidates bind to N. gonorrhoeae strains and promote serum bactericidal and opsonophagocytic activities. This work is supported by the Gonococcal Vaccine Cooperative Research Center led by Dr. Ann Jerse of Uniformed Services University of the Health Sciences and a human clinical trial led by Drs. Alex Duncan and Marcia Hobbs of University of North Carolina-Chapel Hill.