Mechanisms of Sudden Death in Epilepsy (SUDEP)
Overview
The Patel laboratory has recently begun to use the mouse models of SCN8A epileptic encephalopathy to study mechanisms of SUDEP. SUDEP is defined as the sudden, unexpected, and unexplained death of a person with epilepsy, for which a postmortem examination does not produce a cause of death. SUDEP accounts for between 8 and 17% of all epilepsy-related deaths, rising to 50% in for those with seizures that are refractory to treatment. Amongst all neurological conditions, it is second only to stroke for number of life-years lost. Increasing evidence supports apnea (breathing cessation) as the primary cause of death following a seizure. A better understanding of the key processes involved in respiratory dysfunction and subsequent SUDEP would allow for the development of novel rescue therapies. Patients with SCN8A epileptic encephalopathy (EE) have a high incidence of SUDEP.
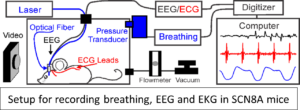 Using our two mouse models of SCN8A epileptic encephalopathy we have developed a system to chronically record cardiac and breathing function while simultaneously monitoring cortical seizure activity. We have recently added functionality for hippocampal stimulation of seizures, brainstem DC recordings, and optogenetic stimulation.
Using our two mouse models of SCN8A epileptic encephalopathy we have developed a system to chronically record cardiac and breathing function while simultaneously monitoring cortical seizure activity. We have recently added functionality for hippocampal stimulation of seizures, brainstem DC recordings, and optogenetic stimulation.
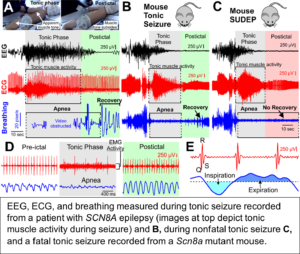 Our recent studies demonstrate that apnea and bradycardia observed during the tonic phase of seizures from a patient with SCN8A epileptic encephalopathy are nearly identical to those observed during spontaneous seizures in two Scn8a mutant mouse models. In the mice, these seizures proved fatal when breathing did not resume at the end of the tonic phase, indicating breathing, not cardiac, dysfunction is the primary cause of seizure-induced death. We also utilized a new technique to record diaphragm EMG during seizures and demonstrated that tonic diaphragm contraction occurs during the tonic phase.
Our recent studies demonstrate that apnea and bradycardia observed during the tonic phase of seizures from a patient with SCN8A epileptic encephalopathy are nearly identical to those observed during spontaneous seizures in two Scn8a mutant mouse models. In the mice, these seizures proved fatal when breathing did not resume at the end of the tonic phase, indicating breathing, not cardiac, dysfunction is the primary cause of seizure-induced death. We also utilized a new technique to record diaphragm EMG during seizures and demonstrated that tonic diaphragm contraction occurs during the tonic phase.
In a related study we demonstrated that immediate injection with an alpha1-adrenergic receptor agonist, phenylephrine, can promote breathing recovery and prevent death. Conversely, at older developmental timepoints seizures were normally survived; however, when we inject mice with an alpha1-adrenergic receptor antagonist, recovery breathing was inhibited and seizures became fatal. Finally, we found that mechanical ventilation could promote survival even in the absence of functional adrenergic signaling, indicating a role in promotion in recovery breathing.
Ongoing and Future Projects
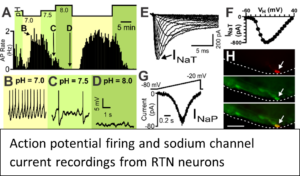 Our current working hypothesis is that SUDEP occurs in mice when breathing ceases during the tonic phase of a seizure, as a result of constant tonic inspiratory activity, and failure of breathing recovery is due to impaired cardiorespiratory homeostasis. We propose that the inspiratory oscillator is tonically active during apnea and we will determine the role of the Bötzinger complex (BötC) and retrotrapezoid nucleus (RTN) brainstem neurons on
Our current working hypothesis is that SUDEP occurs in mice when breathing ceases during the tonic phase of a seizure, as a result of constant tonic inspiratory activity, and failure of breathing recovery is due to impaired cardiorespiratory homeostasis. We propose that the inspiratory oscillator is tonically active during apnea and we will determine the role of the Bötzinger complex (BötC) and retrotrapezoid nucleus (RTN) brainstem neurons on 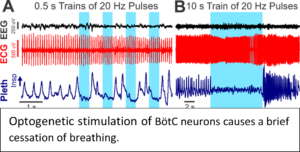 coordinating inspiratory activity during seizure-induced apnea using optogenetic techniques.
coordinating inspiratory activity during seizure-induced apnea using optogenetic techniques.
Epilepsy patients at risk for SUDEP have impaired central chemosensitivity. We propose to assess in vivo CO2-sensitivity at developmental time points leading up to SUDEP and determine if inhibition of sodium channel (INa) currents can rescue CO2-sensitivity. We will determine changes in RTN and local inhibitory neurons to determine their CO2/H+-sensitivity, intrinsic excitability, and INa currents. Finally, we will use shRNA to knockdown Nav1.6 in the RTN and assess its contribution to in vivo CO2-sensitivity and SUDEP.
Altered autonomic control of the heart is a proposed contributor of SUDEP, and we and others find that bradycardia occurs immediately prior to SUDEP in the Scn8a mutant mice. We will determine in vivo parasympathetic cardiac drive leading up to SUDEP and determine effects of sodium channel inhibition. We will make recordings from parasympathetic cardiovagal neurons and determine the effects of Nav1.6 knockdown on bradycardia and SUDEP. These studies will significantly impact our current understanding of the cardiorespiratory alterations that lead to SUDEP and could provide important insight into novel therapeutic targets to prevent SUDEP.
