In-Vivo Cancer Therapeutic Testing Services
The MITS Core offers comprehensive services to run the preclinical testing of anti-cancer therapies in cancer xenograft models or in syngeneic rodent models (mouse and rat), from start to finish. Briefly, these services include tumor implantation and growth, tumor treatment with your anti-cancer therapy, monitoring of tumor size by imaging or caliper measurements, monitoring of rodent health and survival, and tumor and tissue harvest. A few of the available models and services are described below and are fully customizable to fit your study’s needs.
Common Services Used for Testing Anti-Cancer Therapies in Rodents
- Cancer cell engraftment/tumor implantation in rodent xenograft or syngeneic model
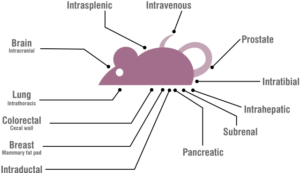
- Administration of therapeutics by gavage, intra-tumorally, subcutaneously, intra-peritoneally, intra-venously, osmotic pump, food, or water. Commonly administered therapeutics include small molecules, biopharmaceuticals, and vaccines.
- Maximum tolerated dose evaluations of single and combined therapies and treatment regimens
Pharmacokinetic drug studies: We offer blood sampling at sequential time points following single- and multi-dose treatment regimens. - Treatment Efficacy Studies: We offer cancer implantation, tumor and health evaluations, weight monitoring, administration of therapeutics, sample collection, survival tracking.
- Collection of tumor growth data by caliper measurements, and/or bioluminescence imaging support
Additional murine surgical services: castration, oophorectomy, and thyroidectomy - Training of Staff to perform these studies
Common Mouse Models Used for Testing Anti-Cancer Therapies
- Syngeneic model: Tumor cell lines from a mouse genetic background are implanted into a mouse with an intact native immune system. These models provide an effective approach for studying how cancer therapies perform in the presence of a functional immune system. They can be customized to use genetically modified mouse tumor cells.
- Cancer xenograft mouse model: A cancer xenograft is a standard model widely used for the testing of anti-cancer therapies. The MITS Core offers services utilizing 3 types of Xenograft models described below. Conventional xenograft models are performed in immunocompromised mice; thus, studies of immune cells are more limited in this model. If needed for your study, the MITS Core can also perform transfers of purified immune cell subsets into mice.
-
Cell line-derived xenograft (CDX model): Human tumor samples are cultured as cell lines and implanted subcutaneously into mice to test the efficacy of an anti-tumor compound/s in vivo.
- Orthotopic model xenograft: Human tumor cells or freshly resected tumor pieces are deposited into the anatomical location of origin in mice/rodents to test the efficacy of an anti-tumor compound/s in vivo.
- Metastasis model xenograft: Cancer cells are deposited into mice for the purpose of developing a primary growth and a secondary malignant growth in a distant location.
*Investigators will provide cells for tumor implantation models.
Image: Charles River Laboratories
Efficacy evaluation in Xenograft models can be combined with:
- Histopathology or tissue sampling
- Analysis of biomarkers or immune infiltrates in tissues with multiplex Immunofluorescence histology
Biomarker screening, sampling, or evaluation - Analysis of biomarkers with flow cytometric analysis
- Multiplex ELISA assays on murine blood
- In vivo implantable microdevices; simultaneous in vivo testing of multiple drugs, drug doses, or drug combinations in a single tumor
For questions regarding preclinical rodent study services, please contact: Marya Dunlap-Brown at 434-243-6505 or med4s@virginia.edu.
Publications Utilizing Preclinical Rodent Services
Periostin + stromal cells guide lymphovascular invasion by cancer cells. Null JL, Kim DJ, McCann JV, Kumar P, Edatt L, Pecot CV, Dudley, AC. DOU: doi.org/10.1101/2022.05.19.492742. Preprint on bioRxiv.org.
Credentialing and Pharmacologically Targeting PTP4A3 Phosphatase as a Molecular Target for Ovarian Cancer. Lazo, J.S.; Sharlow, E.R.; Cornelison, R.; Hart, D.J.; Llaneza, D.C.; Mendelson, A.J.; Rastelli, E.J.; Tasker, N.R.; Landen, C.N., Jr.; Wipf, P. Biomolecules, 11, 969, 2021. DOI: 10.3390/biom11070969.
Whole Genome Sequencing of Spontaneously Occurring Rat Natural Killer Large Granular Lymphocyte Leukemia Identifies JAK1 Somatic Activating Mutation. TT Wang, J Yang, S Dighe, MW Schmachtenberg, NT Leigh, E Farber, S Onengut-Gumuscu, DJ Feith, A Ratan, TP Loughran, and TL Olson. Cancers, 12(1), 126, 2020. DOI: 10.3390/cancers12010126
Deletion of the SAPS1 subunit of protein phosphatase 6 in mice increases radiosensitivity and impairs the cellular DNA damage response. Jaroslaw Dziegielewski, Magdalena A. Bońkowska, Ewa A. Poniecka, Jinho Heo, Kangping Du, Rowena B. Crittenden, Timothy P. Bender, David L. Brautigan, James M. Larner. DNA Repair Vol 85, 2020. DOI: 10.1016/j.dnarep.2019.102737