Mark D. Okusa, MD, FASN, Division Chief
PRIMARY APPOINTMENT

John C. Buchanan Distinguished Professor of Medicine: Nephrology
Contact
UVA Division of Nephrology
PO Box 800133
Charlottesville, VA 22908
Telephone: 434-924-2187
Fax: 434-982-5575
Email: mdo7y@virginia.edu
EDUCATION AND TRAINING
- MD, Medical College of Virginia/Virginia Commonwealth University
- Residency, Medical College of Virginia/Virginia Commonwealth University
- Fellowship, Yale School of Medicine
FUNDED PROJECTS
- R01DK105133 (Ultrasound for Non-Invasive Prevention of Acute Kidney Injury)
- NIH/NIDDK 1R01DK123248
- (Neuroimmune Regulation of Acute Kidney Injury)
- R01 DK085259 (Sphingolipids in Acute Kidney Injury and Disease Progression)
- R01 DK123248 (Neuroimmune Regulation of Acute Kidney Injury)
- T32 DK072922 (Kidney Disease and Inflammation)
RESEARCH AREAS
- Inflammation, fibrosis, progression of kidney disease
CURRENT LABORATORY MEMBERS
 CONTACT:
CONTACT:
Email: sc9d@virginia.edu
Dr. Sylvia Cechova received her undergraduate degree in Nuclear Chemistry as well as her graduate Ph.D. degree in Radiation Chemistry from Comenius University in Bratislava, Slovakia. She conducted the postdoctoral research in the Department of Anesthesiology and in the Department of Chemistry, University of Virginia, to study the effect of drugs and anesthetics on the threshold for isoflurane anesthesia, and to study the transient release of adenosine and dopamine in rat brain in vivo, using fast-scan voltammetry with carbon-fiber microelectrode, respectively. She joined the Department of Medicine, Division of Nephrology in August 2013 to work with Dr. Thu Le on the project that focused on the identification of genes associated with hypertension. Especially, they were interested in GSTM1 and Collectrin genes, and how the deletion or modification of them will affect the development of hypertension and progression of kidney disease, and its cardiovascular consequences. After the last 2 years in Dr. Swaminatan Lab, where she focused on the roles of hepcidin, ferroptosis, and macrophage iron metabolism in the progression of chronic kidney disease and renal fibrosis, she joined the Dr. Okusa and Dr. Lobo labs in May 2020. The focus of her current research is the contribution of sphingolipid pathway on prevention or attenuation of fibrosis following AKI and the role of natural IgM upregulation in the recovery after AKI.
 Eibhlin (Ebby) Goggins graduated from Georgetown University in 2018 with a BS in Biochemistry. She spent four years in Dr. Zaver Bhujwalla’s lab at Johns Hopkins University studying the role of hypoxia-inducible factors in breast cancer. Ebby also completed a senior thesis investigating the mechanisms of action of antimalarial drugs in Dr. Paul Roepe’s lab.
Eibhlin (Ebby) Goggins graduated from Georgetown University in 2018 with a BS in Biochemistry. She spent four years in Dr. Zaver Bhujwalla’s lab at Johns Hopkins University studying the role of hypoxia-inducible factors in breast cancer. Ebby also completed a senior thesis investigating the mechanisms of action of antimalarial drugs in Dr. Paul Roepe’s lab.
In 2019, she entered the MSTP at the University of Virginia. In 2021, after discovering her love for the kidneys, she joined the Okusa Lab as a PhD student. Her first project investigates using pulsed ultrasound to prevent and treat kidney disease. Her studies involve understanding the mechanism behind this kidney protection and expanding the use of ultrasound to additional models of kidney disease. Her second project focuses on mitochondrial dynamics, specifically the role of the mitochondrial fission protein, Drp1, in kidney perivascular cells.
After completing her PhD, Ebby will return to medical school to finish her clinical rotations. Ultimately, she hopes to pursue a career as a physician-scientist in nephrology.
Email: EG8YR@uvahealth.org
 Shuhei obtained PhD in Pharmacy from Chiba University, Japan. In his doctorate studies, he explored the role of metabolic regulation by Notch signaling pathway in embryonic development and treatments for glioblastoma. He joined Okusa lab in April 2021 as a postdoctoral fellow and is working on basic studies on acute kidney injury. His main project dissects the relationship between Notch signaling in macrophages and cholinergic anti-inflammatory pathway that protects the kidneys against acute kidney injury.
Shuhei obtained PhD in Pharmacy from Chiba University, Japan. In his doctorate studies, he explored the role of metabolic regulation by Notch signaling pathway in embryonic development and treatments for glioblastoma. He joined Okusa lab in April 2021 as a postdoctoral fellow and is working on basic studies on acute kidney injury. His main project dissects the relationship between Notch signaling in macrophages and cholinergic anti-inflammatory pathway that protects the kidneys against acute kidney injury.
 CONTACT:
CONTACT:
Email:
William (Billy) obtained his BS in biology from Gonzaga University in Washington State and then crossed the US to attend the University of Virginia for a PhD in Microbiology/Immunology. His doctoral studies centered around the effects of inflammation and innate immune system-mediated virus control on dendritic cell (DC) populations. His findings challenged a long-standing paradigm in the field and showed that type I interferon regulation of DC subsets is dynamic, complex, and subset-specific. Due to his work with a local non-profit and other interests in the area, he opted to remain in Charlottesville after completing his PhD. He joined the division of Nephrology at UVA in 2016 to investigate immune mechanisms that promote survival or mortality during life-threatening inflammatory disease, using sepsis as a model. His current work is focused on uncovering the role of macrophages in the deterioration or preservation of kidney function during sepsis. Macrophages play key roles in both inflammatory and anti-inflammatory pathways and his projects are designed to dissect these opposing functions.
In his spare time, he enjoys the company of his friends and going to wineries, movies, amusement parks, on hikes, or just out around town. He also enjoys a good book or crossword puzzle in his downtime.

Shinji Tanaka, MD, PhD
Postdoctoral Fellow
CONTACT:
Email: st3ff@virginia.edu
Shinji obtained his MD from the University of Tokyo, Japan, and then worked as a physician/nephrologist for 6 years in Japan. Subsequently, he went back to the University of Tokyo to get his PhD. In his doctorate studies, he explored the renoprotective effect of SGLT2 inhibitors and PHD inhibitors in diabetic kidney disease as well as immune cell infiltration in acute kidney injury. He joined Okusa lab in 2016 as a postdoctoral fellow and is working on basic studies on acute kidney injury and chronic kidney disease. His first project deals with dissecting the neural circuits that are involved in the renoprotection by vagus nerve stimulation. His other major project is to investigate the role of sphingosine-1-phosphate in the progression of kidney fibrosis.

Junlan Yao, PhD
Assistant Professor of Research
CONTACT:
Telephone: 434-924-2127
Email: jy9n@virginia.edu

Shuqiu Zheng, MD
Senior Research Scientist
CONTACT:
Email: zs6n@virginia.edu
Shuqiu obtained her MD in Bethune University of Medical Sciences and PhD in the Department of Pharmacology, Sun Yat-Sen University of Medical Sciences in China. She has been doing basic science research at UVA from 2001 until the present time. Currently in Dr. Okusa’s lab, she is an expert in performing mouse kidney ischemia as a model of AKI. In addition, her research interest is in using photoacoustic microscopy, a new technique that provides real-time dynamic monitoring of renal peritubular capillary O2 tension and blood flow in LPS-induced AKI. She hopes to develop new ideas and discoveries about the effects of US on oxygen stress in LPS induced kidney sepsis.
Lab Team Images
RESEARCH SUMMARY
Immune Mechanisms of Acute Kidney Injury. We have focused our attention on the immunopathogenesis of acute kidney injury (AKI). We have demonstrated the important role that immune cells play in the pathogenesis of AKI. We have described the role of dendritic cells, natural killer T cells, neutrophils, macrophages, and T regulatory cells (Tregs). These studies have demonstrated that early in the course of ischemia-reperfusion injury there is the activation of dendritic cells, which leads to glycolipid presentation to natural killer T cells. With our knowledge of the immunopathogenesis of AKIwe have been engaged in preclinical studies to characterize the mechanism of action of leading compounds targeted to AKI.
Microenvironment in AKI and AKI to CKD Transition. Our interest in the microenvironment began with our studies with adenosine receptors. Adenosine 2a receptors (A2aR) belong to a class of G-protein coupled receptors comprised of A1, A2a, A2b, A3 receptors. We have studied the role of agonists that bind to A2aR. Administration of an A2aR agonist markedly reduced AKI. We observed that activation ofA2aRs in dendritic cells, CD4 cells, and T regulatory cells is critically important in contributing to the efficacy of this class of compounds. This compound has important implications not only in systemic administration but also in cell-based therapy. More recently we observed that ATP release channels(Pannexin 1) contribute to AKI through the control of intracellular ATP or through its release into the microenvironment. Whereas much is known about the extracellular role of ATP in activating inflammation, less is known regarding the role of Pannexin 1 channels in controlling intracellular ATP that leads to protection from AKI.
Sphingolipids in Kidney Disease. A second target that we have focused our attention on is the sphingolipid pathway. Sphingosine 1-phosphate is the natural ligand for five G-protein coupled receptors, S1P1-5. Binding to one of these receptors, S1P1, has potent effects to block IRI. Using Cre-Lox technology we have determined that S1P1 in proximal tubule cells, dendritic cells, and endothelial cells contribute importantly to the protective effect of S1P1 compounds. Our studies also examine the role of sphingosine kinase2 in pericytes as a major factor in the progression of kidney disease. These studies undergird the development of lead sphingosine kinase 2 inhibitors for attenuation of fibrosis following AKI.
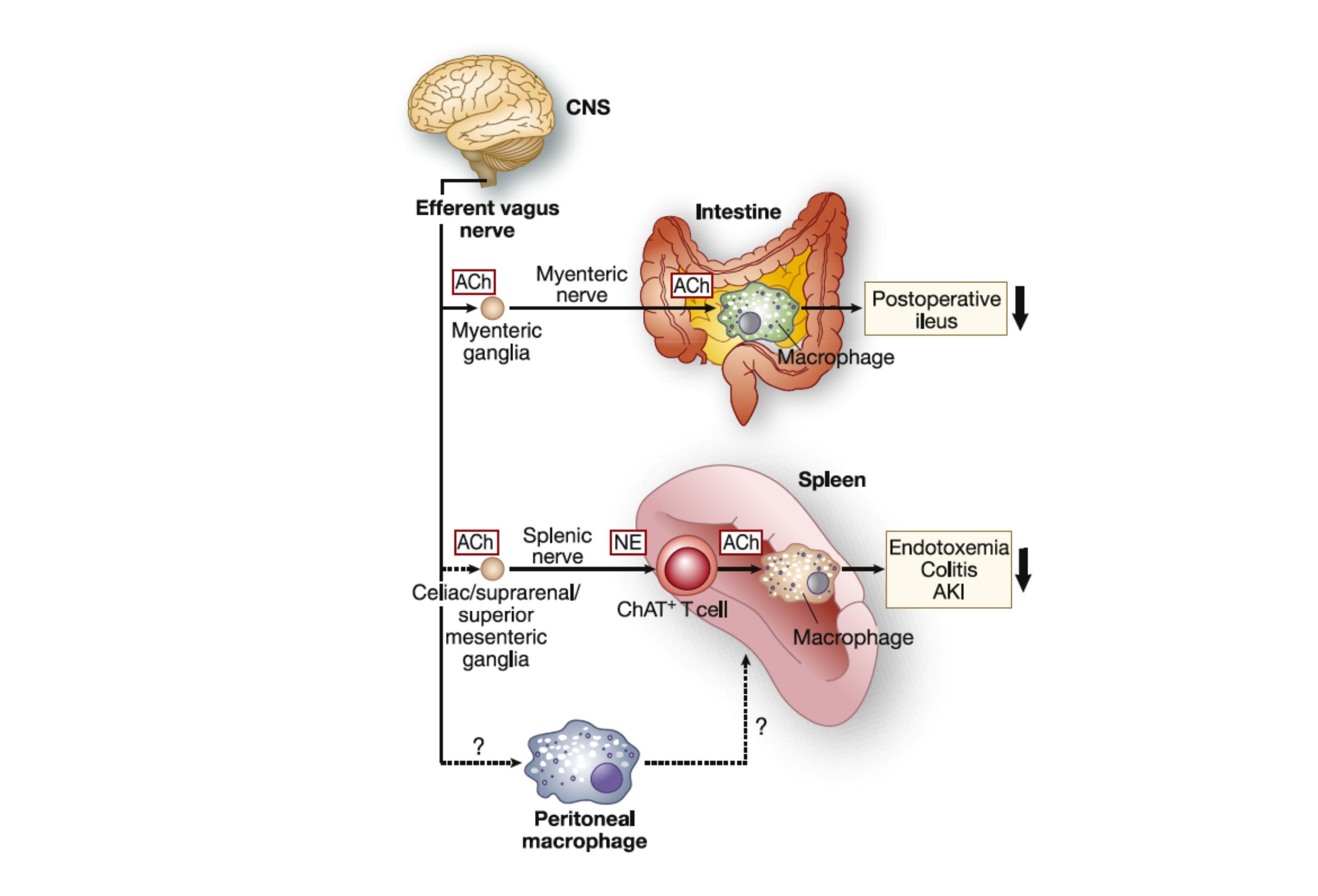
Neuroimmunomodulation in Acute Kidney Injury. We study the inflammatory reflex, a neuro-immune circuit that is critical for immunological homeostasis. The efferent limb of the inflammatory reflex pathway is referred to as the cholinergic anti-inflammatory pathway (CAP), and the CAP is initiated via activation of the catecholaminergic splenic nerve and release of norepinephrine and culminates with activation of α7-nicotinic acetylcholine receptors. We have focused on activating this pathway through novel approaches including electrical vagus nerve stimulation, optogenetics, and pulsed ultrasound. These results suggest that the CAP is important in modulating AKI and that the nonpharmacological application of ultrasound using parameters within the FDA guidelines may protect kidneys from IRI. Our studies using optogenetics allow precise characterization of the neural circuitry that regulates inflammation.
PUBLICATION TREEMAP FOR MARK OKUSA
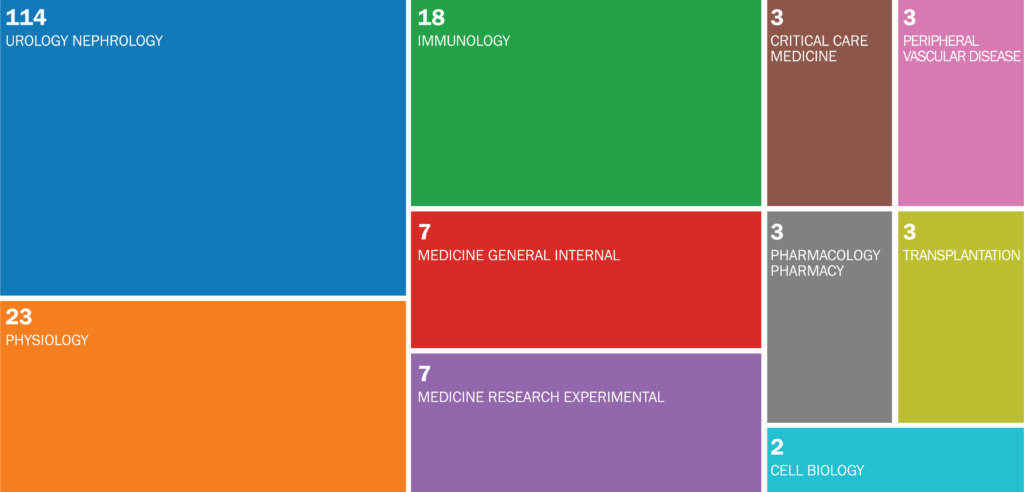
114 Urology Nephrology Publications, 23 Physiology Publications, 18 Immunology Publications, 7 Medicine General Internal Publications, 7 Medicine Research Experimental Publications, 3 Critical Care Medicine Publications, 3 Pharmacology Pharmacy Publications, 3 Peripheral Vascular Disease Publications, 3 Transplantation Publications, 2 Cell Biology Publications