Presynaptic Fusion
Membrane Fusion in Neurotransmitter Release
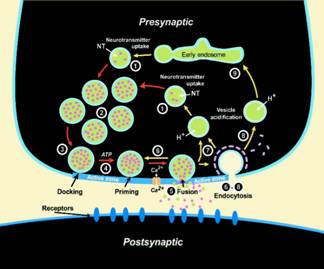
The ‘SNARE hypothesis’ states that neurotransmitter-loaded secretory vesicles fuse and release their contents in milliseconds with presynaptic membranes by zippering v-SNAREs on vesicle membranes and t-SNAREs on target membranes into a 4-helix coiled coil structure. How the force of this highly exothermic reaction is transmitted into deforming membranes and how the fusion triggers calcium and synaptotagmin regulate this process is unclear and a major focus of our research.
In a large collaborative effort (NIH program project) with the groups of Reinhard Jahn at the Max-Planck Institute in Göttingen, Germany, David Cafiso in the Chemistry Department at UVA, and David Castle in the Department of Cell Biology at UVA, we are taking a multi-pronged cell-biological, biochemical, and biophysical approach to this problem. We are studying the structures of the relevant fusion proteins by combined NMR and EPR approaches in membranes.
High-resolution structural information of individual components and domains is then integrated into the understanding of this multi-component molecular machine by single molecule fluorescence studies. By reconstitution of the relevant components in supported bilayers, single fusion events can be observed at millisecond time resolution and analyzed in terms of various functional models. FRET experiments permit us to determine changing spatial relationships of protein and lipid components in this process. Similar studies with native plasma membranes of secretory cells and synaptic vesicles allow us to link the reconstitution approach with the cell physiology of this process.
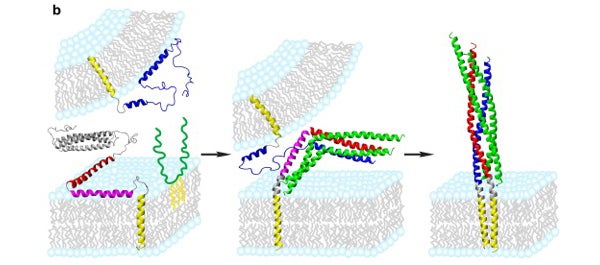
Models of SNARE assembly in presynaptic membrane fusion based on perfusion NMR structures, postfusion crystal structure, fluorescence interference contrast (FLIC) microscopy and single molecule studies
- Vesicle cycling at a synapse.
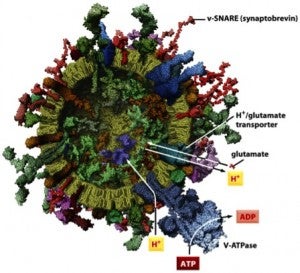
- Structures of proteins involved in intracellular vesicle fusion.
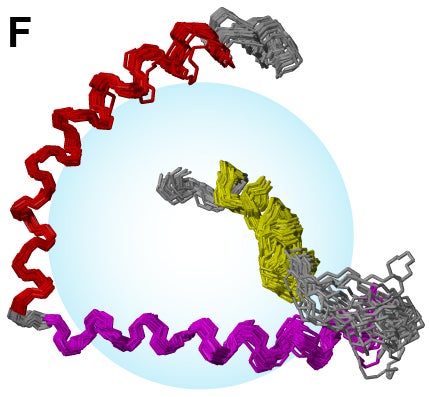
- NMR structures of synaptic fusion protein synaptobrevin.
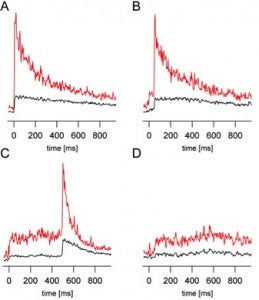
- Signals from individual synaptic vesicle fusion events.
- Synaptic vesicle.
- Prefusion NMR structures of synaptic t-SNARE syntaxin in DPC micelle
**click to view full size image
Recent Key Publications:
Jahn R, Cafiso DS, Tamm LK (2023). Mechanisms of SNARE proteins in membrane fusion. Nature Rev Cell Mol Biol. Epub ahead of print. doi: 10.1038/s41580-023-00668-x.
Coffman RE, Kraichely KN, Kreutzberger AJB, Kiessling V, Tamm LK, Woodbury DJ (2022). Drunken lipid membranes, not drunken SNARE proteins, promote fusion in a model of neurotransmitter release. Front Mol Neuroscience 15:1022756. doi: 10.3389/fnmol.2022.1022756. (https://pubmed.ncbi.nlm.nih.gov/36311016/)
Liang Q, Ofosuhene AP, Kiessling V, Liang B, Kreutzberger AJB, Tamm LK, Cafiso DS (2022) Complexin-1 and synaptotagmin-1 compete for binding sites on membranes containing PtdInsP2. Biophys J 121:3370-3380. (https://pubmed.ncbi.nlm.nih.gov/36016497/)
Nyenhuis SB, Karandikar N, Kiessling V, Kreutzberger AJB, Thapa A, Liang B, Tamm LK and Cafiso DS (2021) Conserved arginine residues in synaptotagmin 1 regulate fusion pore expansion through membrane contact. Nature Commun. doi: 10.1038/s41467-021-21090-x. (https://pubmed.ncbi.nlm.nih.gov/33536412/)
Kreutzberger AJB, Kiessling V, Stroupe C, Liang B, Preobraschenski J, Ganzella M, Kreutzberger MAB, Nakamoto R, Jahn R, Castle JD, Tamm LK. In vitro fusion of single synaptic and dense core vesicles reproduces key physiological properties. Nat Commun. 2019 Aug 29;10(1):3904. doi: 10.1038/s41467-019-11873-8. (https://www.ncbi.nlm.nih.gov/pubmed/31467284)
Brose N, Brunger A, Cafiso D, Chapman ER, Diao J, Hughson FM, Jackson MB, Jahn R, Lindau M, Ma C, Rizo J, Shin YK, Söllner TH, Tamm L, Yoon TY, Zhang Y. Synaptic vesicle fusion: today and beyond. Nat Struct Mol Biol. 2019 Aug;26(8):663-668. doi: 10.1038/s41594-019-0277-z. (https://www.ncbi.nlm.nih.gov/pubmed/31384060)
Kiessling V, Kreutzberger AJB, Liang B, Nyenhuis SB, Seelheim P, Castle JD, Cafiso DS, Tamm LK. A molecular mechanism for calcium-mediated synaptotagmin-triggered exocytosis. Nat Struct Mol Biol. 2018 Oct;25(10):911-917. doi: 10.1038/s41594-018-0130-9. Epub 2018 Oct 5. (https://www.ncbi.nlm.nih.gov/pubmed/30291360)
Liang B, Tamm LK. Solution NMR of SNAREs, complexin and α-synuclein in association with membrane-mimetics. Prog Nucl Magn Reson Spectrosc. 2018 Apr;105:41-53. doi: 10.1016/j.pnmrs.2018.02.001. Epub 2018 Feb 8. (https://www.ncbi.nlm.nih.gov/pubmed/29548366)
Kreutzberger AJB, Kiessling V, Liang B, Yang ST, Castle JD, Tamm LK. Asymmetric Phosphatidylethanolamine Distribution Controls Fusion Pore Lifetime and Probability. Biophys J. 2017 Oct 13. pii: S0006-3495(17)31026-3. doi: 10.1016/j.bpj.2017.09.014. [Epub ahead of print](https://www.ncbi.nlm.nih.gov/pubmed/29037600)
Kreutzberger AJB, Kiessling V, Liang B, Seelheim P, Jakhanwal S, Jahn R, Castle JD, Tamm LK. Reconstitution of calcium-mediated exocytosis of dense-core vesicles. Science Advances 19 Jul 2017: Vol. 3. No. 7. E1603208 DOI:10.1126/sciadv.1603208 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5517108/)
Zdanowicz R, Kreutzberger A, Liang B, Kiessling V, Tamm LK, Cafiso DS. Complexin Binding to Membranes and Acceptor t-SNAREs Explains Its Clamping Effect on Fusion. Biophys J. 2017 Sep 19;113(6):1235-1250. (https://www.ncbi.nlm.nih.gov/pubmed/28456331)
Kiessling V, Liang B, Kreutzberger AJ, Tamm LK. Planar Supported Membranes with Mobile SNARE Proteins and Quantitative Fluorescence Microscopy Assays to Study Synaptic Vesicle Fusion. Front Mol Neurosci. 2017 Mar 16;10:72. doi: 10.3389/fnmol.2017.00072. eCollection 2017. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5352703/)
Yang ST, Kreutzberger AJ, Lee J, Kiessling V, Tamm LK. The role of cholesterol in membrane fusion. Chem Phys Lipids. 2016 Sep;199:136-43. doi: 10.1016/j.chemphyslip.2016.05.003. Epub 2016 May 11. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4972649/)
Kreutzberger AJ, Liang B, Kiessling V, Tamm LK. Assembly and Comparison of Plasma Membrane SNARE Acceptor Complexes. Biophys J. 2016 May 24;110(10):2147-50. doi: 10.1016/j.bpj.2016.04.011. Epub 2016 May 10. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4881159/)
Kreutzberger AJ, Kiessling V, Tamm LK. High cholesterol obviates a prolonged hemifusion intermediate in fast SNARE-mediated membrane fusion. Biophys J. 2015 Jul 21;109(2):319-29. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4621810/)
Kiessling V, Liang B, Tamm LK. Reconstituting SNARE-mediated membrane fusion at the single liposome level. Methods Cell Biol. 2015 ;128:339-63. ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4443872/)
Hernandez, J.M., Kreutzberger, A.J., Kiessling, V., Tamm, L.K., Jahn, R. Variable cooperativity in SNARE-mediated membrane fusion. Proc Natl Acad Sci USA. 2014 Aug 19;111(33):12037-42 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4143004/)
Lu B, Kiessling V, Tamm LK, Cafiso DS. The juxtamembrane linker of full-length synaptotagmin 1 controls oligomerization and calcium-dependent membrane binding. J Biol Chem. 2014 Aug 8;289(32):22161-71 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4139229/)
Liang, B., Dawidowski, D., Ellena, J.F., Tamm, L.K., Cafiso, D.S. The SNARE Motif of Synaptobrevin Exhibits an Aqueous-Interfacial Partitioning That Is Modulated by Membrane Curvature. Biochemistry 2014 Mar 11;53(9):1485-94. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3985803/)
Liang, B., Kiessling, V., Tamm, L.K. Prefusion structure of syntaxin-1A suggests pathway for folding into neuronal trans-SNARE complex fusion intermediate. Proc Natl Acad Sci U S A. 2013 Nov 26;110(48):19384-9. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3845119/)
Kiessling, V., Ahmed, S., Domanska, M., Holt, M., Jahn, R., Tamm, L. Rapid Fusion of Synaptic Vesicles with Reconstituted Target SNARE Membranes. Biophysical Journal 2013 May 7;104(9):1950-8. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3647153/)
Lai, A.L., Tamm, L.K., Ellena, J.F., Cafiso, D.S. Synaptotagmin 1 modulates lipid acyl chain order in lipid bilayers by demixing phosphosphatidylserine. J. Biol. Chem. 2011 Jul 15;286(28):25291-300 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3137100/)
Murray, D., and Tamm, L.K. Molecular mechanism of cholesterol- and phosphoinositide-mediated syntaxin clustering. Biochemistry 2011 Oct 25;50(42):9014-22. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3199950/)
Wan, C., Kiessling, V., Cafiso, D.S., Tamm, L.K. Partitioning of synaptotagmin I C2 domains between liquid-ordered and liquid-disordered inner leaflet lipid phases. Biochemistry 2011 Apr 5;50(13):2478-85. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3094915/)
Domanska, M.K., Kiessling, V., Tamm,L.K. Docking and fast fusion of synaptobrevin vesicles depends on lipid compositions of the vesicle and the acceptor SNARE complex-containing target membrane. Biophys. J. 2010 Nov 3;99(9):2936-46 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2965956/)
Kiessling, V., Domanska, M.K., and Tamm,L.K. Single SNARE-mediated vesicle fusion observed in vitroby polarized TIRFM. Biophys. J. 2010 Dec 15;99(12):4047-55. ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000493/)
Ellena, J.F., Liang B., Wiktorb, M., Stein, A., Cafiso, D.S., Jahn, R., Tamm, L.K. Dynamic structure of lipid-bound synaptobrevin suggests a nucleation-propagation mechanism for trans-SNARE complex formation. PNAS 2009 Dec 1;106(48):20306-11. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2787132/)
Domanska, M.K., Kiessling, V., Stein, A., Fasshauer, D., Tamm, L.K. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J Biol Chem 2009 Nov 13;284(46):32158-66. ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2797286/)
Murray, D. H., Tamm, L. K. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry 2009 Jun 2;48(21):4617-25. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2724070/)
Tamm, L.K., J. Crane, and V. Kiessling. Membrane fusion: a structural perspective on the interplay of lipids and proteins. (Review) Curr. Op. Struct. Biol. 2003 Aug;13(4):453-66. (http://www.ncbi.nlm.nih.gov/pubmed/12948775)
Kiessling, V. and L.K. Tamm Measuring distances in supported bilayers by fluorescence interference-contrast microscopy: polymer supports and SNARE proteins. Biophys. J. 2003 Jan;84(1):408-18.(http://www.ncbi.nlm.nih.gov/pubmed/12524294)
Wagner, M.L. and L.K. Tamm Reconstituted syntaxin1A/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J. 2001 Jul;81(1):266-75. (http://www.ncbi.nlm.nih.gov/pubmed/11423412)