Research
Investigating the cellular and molecular mechanisms underlying host pathogen interaction
Our laboratory investigates the cellular and molecular mechanisms underlying host pathogen interaction. Our research efforts are divided in two related areas. First, we have pioneered and perfected innovative genetic approaches to systematically interrogate the human genome and determine the host genes involved in a given cellular process. Second, we have applied our expertise in large-scale genetic approaches to uncover the genes and related cellular processes supporting intracellular pathogen infection.
Genetic investigations using RNA interference
RNA interference (RNAi) is a cellular mechanism that mediates the sequence-specific destruction of RNA molecules in response to the introduction of homologous double-stranded RNA (dsRNA). The discovery of RNAi revolutionized genetic studies by providing a powerful approach for gene silencing. This is achieved through transfection (or expression) of dsRNA displaying sequence homology with the mRNA of a targeted gene. The availability of dsRNA library covering the whole human genome, in combination with high-throughput screening approaches, automated microscopy and computer-assisted image analysis, empowered us with the ability to determine, on a genome-wide scale, the set of genes involved in a given cellular process. In our laboratory, we utilize the approach to identify the set of genes supporting intracellular pathogen infection. The identified genes, and their cellular functions, define the mechanisms supporting host pathogen interaction and therefore suggest potential targets for therapeutic interventions.
Mechanisms supporting intracellular pathogen infection
Our laboratory is currently investigating the mechanisms supporting the development and propagation of Shigella flexneri and Listeria monocytogenes in the human intestine. These unrelated bacterial pathogens invade intestinal cells and gain access to the cytosolic compartment, where they manipulate the host cell actin cytoskeleton and display actin-based motility. As they reach the cell periphery, motile pathogens form plasma membrane protrusion that project into adjacent epithelial cells, where protrusions resolve into vacuoles. As the pathogens escape from the formed vacuoles, they gain access to the cytosol of adjacent cells, thereby achieving cell-to-cell spread. In the past few years, using RNAi-based genetic approaches, we have systematically explored the host cell genes supporting pathogen spread from cell to cell. In addition to the genes coding for the factors required for reconstitution of actin-based motility in vitro, our genetic studies in intestinal cells led to the identification of genes required for efficient intracellular motility in vivo (Chong et al., 2009; Chong et al., 2011; Alvarez et al., 2012; Alvarez et al., 2013; Dragoi et al. 2013; Talman et al., 2014). Moreover, we uncovered host cell genes that are not required for actin-based motility, but are essential for efficient cell-to-cell spread. These newly identified genes support the formation and resolution of plasma membrane protrusions at the cell periphery (Talman et al., 2014, Dragoi et al., 2014; Dragoi et al. 2015). Altogether, our comparative studies with Shigella flexneri and Listeria monocytogenes highlight the fascinating notion that, although displaying similar strategies of actin-based motility in the cytosol of infected cells, these intestinal pathogens have evolved strikingly different mechanisms of formation and resolution of membrane protrusions (reviewed in Kuehl et al., 2015). In addition to the study of bacterial pathogens, we also explore the mechanisms supporting action-based motility of viral pathogens, such as vaccinia virus.
Shigella flexneri
We have discovered that Shigella flexneri dissemination relies on the class II lipid kinase PIK3C2A (Dragoi et al., 2015). We demonstrated that (1) PIK3C2A is required for PI(3)P signaling in S. flexneri protrusions, (2) PI(3)P signaling is required for the resolution of protrusion into a newly identified membrane-bound compartment that we refer to as the vacuole-like protrusions (VLPs) and (3) PI(3)P signaling relies on the S. flexneri type III secretion system. We also conducted genetic investigations on the side on the pathogen and discovered the bacterial type III secretion effector required for PI(3)P signaling. In addition to PI(3)P signaling, we have uncovered cellular signaling components and bacterial effector proteins required for the resolution of VLPs into vacuoles, and pathogen escape from the formed vacuoles.
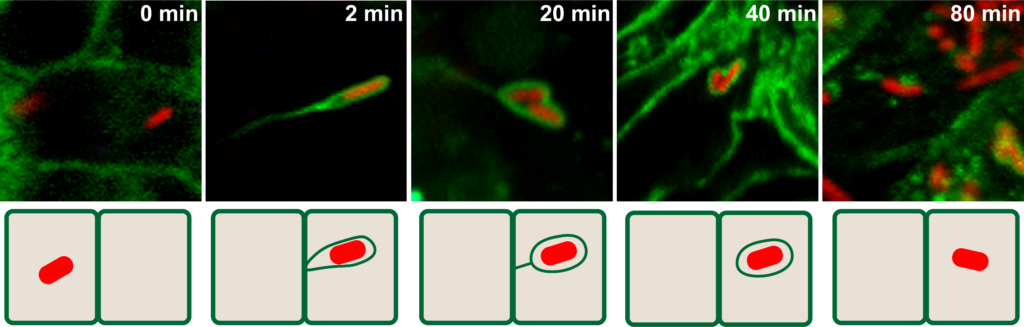
Shigella cell-to-cell spread 0 min – Primary cell: Shigella is in the primary infected cell 2 min – Protrusion: the bacterium protrudes into an adjacent cell 20 min – Vacuole Like Protrusion (VLP): the VLP is still connected to the primary cell by a thin membrane tether 40 min – Vacuole: no longer tethered to primary cell 80 min – Adjacent cell: Shigella has successfully invaded the adjacent cell
Listeria monocytogenes
We have discovered that Listeria monocytogenes dissemination relies on the local recycling of the actin network in protrusions (Talman and Agaisse, 2014). We are currently defining the mechanisms supporting this local recycling process, which includes novel components of the actin cytoskeleton disassembly machinery. We are also investigating the cellular and bacterial factors leading to the resolution of L. monocytogenes protrusions into vacuoles, as our work supports the notion that these mechanisms are different from the mechanisms supporting the resolution of Shigella flexneri protrusions (Kuehl et al., 2015).
Vaccinia virus
We have discovered that vaccinia virus actin-based motility not only relies on the activity of the N-WASP/ARP2/3 pathway, as previously described, but also on the activity of the Rac1/FHOD1 pathway, as published by our group (Alvarez and Agaisse, 2013). We are currently defining the host/pathogen interface supporting the functional integration of these two pathways through the activity of tyrosine kinase signaling components as well as viral components recently identified by our group.
