The role of the cap mesenchyme in establishing nephron number in mice born prematurely
About
 Nearly 500,000 neonates are born prior to the completion of gestation each year in the United States and those born prematurely have an increased risk of developing chronic kidney disease (CKD). A recent, large epidemiologic study revealed infants born weighing <2.5 kg had a 3x higher risk of developing CKD during their childhood . CKD is a widespread, resource-consuming public health epidemic that results from too few nephrons to maintain an individual’s homeostatic balance . The association between
Nearly 500,000 neonates are born prior to the completion of gestation each year in the United States and those born prematurely have an increased risk of developing chronic kidney disease (CKD). A recent, large epidemiologic study revealed infants born weighing <2.5 kg had a 3x higher risk of developing CKD during their childhood . CKD is a widespread, resource-consuming public health epidemic that results from too few nephrons to maintain an individual’s homeostatic balance . The association between
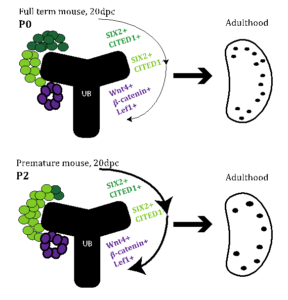
Figure 1. Proposed model. We hypothesize that premature birth (bottom panel) is a signal to convert SIX2+/CITED+ (dark green) to SIX2/CITED1- (light green) depleting the self-renewing population of nephron progenitor cells in the premature mouse kidney resulting in early cessation of nephrogenesis and a reduction of glomeruli.
prematurity and resultant CKD is a culmination of factors unique to the premature infant, including a shortened window of nephron development strained by hemodynamic insults, nephrotoxins, and environmental stressors . Normal nephrogenesis is a process of reciprocal induction between the metanephric mesenchyme and the ureteric bud and is completed at 36 weeks gestation in humans . Within the metanephric mesenchyme are specialized niches of cells that progress from a self-renewing population (SIX2+/CITED1+) to an inducible state (SIX2+/CITED1-) and finally to committed nephrons, allowing nephrogenesis to continue throughout gestation. Little is known about the progression of these cells in the kidney of premature infants following birth. Human studies have demonstrated an abbreviated and accelerated period for premature infants to continue nephrogenesis and the presence of a smaller nephrogenic zone, where nephron progenitors reside.
It is critical to understand the causal pathways between premature birth and CKD, but this area of research has been largely neglected in part due to the lack of an acceptable animal model and the challenges associated with nephron enumeration.Premature baboons studies have been conducted, but these studies are not feasible for most investigators. A recent publication by Stelloh, et al demonstrated that mice delivered 1-2 days prior to the completion of nephrogenesis, developed chronic kidney disease with lower estimated GFR, albuminuria, elevated blood pressure and 20% fewer nephrons by 5 weeks age . This mouse model of prematurity has the potential to shed light on the mechanisms initiated by premature birth and resulting in CKD.
Our long-term goal is to protect and preserve nephrogenesis in infants born prematurely. Therefore, it is imperative to determine the mechanisms leading to the cessation of nephrogenesis and develop techniques to measure nephron endowment. Combining the mouse model of prematurity with our expertise in MRI methods to count nephrons, we will test the following hypothesis(Fig. 1): the cap mesenchyme is more rapidly depleted in mice born prior to the completion of gestation resulting in fewer, larger and abnormally distributed nephrons in adult mice.
