Scientific Theme
About
The main theme of this PCEN is “Regulation of Cell Fate during Kidney Development and Disease.” Its overall goal is to generate novel knowledge for the prevention, management and eventual cure of kidney diseases in children.
The kidney is a highly vascularized organ that in the adult receives about 20% of the cardiac output. The unique spatial arrangement of the kidney arterioles with each nephron is crucial for the regulation of renal blood flow, glomerular filtration rate and other specialized kidney functions that maintain homeostasis. Thus, the proper and timely assembly of the arterioles with their respective nephrons is a crucial morphogenetic event leading to the formation of a functioning kidney necessary for independent extrauterine life. The mechanisms that govern the development and maintenance of the kidney vasculature are poorly understood.
In the US the incidence of Chronic Kidney Disease (CKD) is 16-17% of the adult population. End stage renal disease has been growing at a rate of 8-14% per year and is expected to double during the next 15 years. It is now apparent that many kidney diseases have primary or secondary vascular lesions. Renal failure is frequently associated with defective angiogenesis. Progressive renal disease is characterized by deterioration of the renal microvasculature which correlates with the development of glomerular and tubulointerstitial fibrosis. Alterations in the fate of vascular cells have been identified as a major cause of renal interstitial fibrosis, a situation where pericytes phenotypically switch to become fibroblasts. Vascular rarefaction (and dysfunction) in both small renal and systemic arteries precede renal end-organ damage in models of hypertension-associated renal damage.
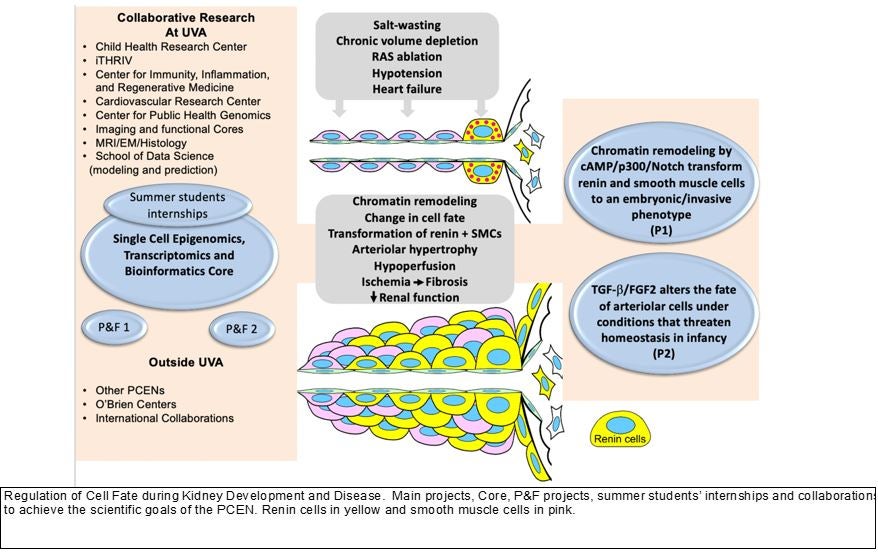
When formation of the kidney vasculature is altered, the consequences are devastating as exemplified by the serious developmental defects that occur in animals and children with mutations of the renin-angiotensin system (RAS) genes and chronic administration of RAS inhibitors (in rodents, monkeys, and humans) resulting in severe and often silent arterial/arteriolar abnormalities that lead to the deterioration of kidney structure and function6, 7. Young children with salt wasting renal diseases are at high risk of developing chronic and severe extracellular fluid volume contraction, leading to growth retardation and poor renal perfusion. Chronic conditions that threaten homeostasis such as hypotension, hypokalemia, and/or salt depletion, as well as the prolonged used of RAS inhibitors in young children induce the recruitment of renin-producing cells with the resulting hypertrophy of the renal arterioles. This response, however, cannot be sustained for too long without leading to the deterioration of renal perfusion and development of renal injury. We have shown that the renin producing cells are progenitors for other vascular types in the kidney including smooth muscle, mesangial cells and pericytes.
Under chronic, relentless stimulation, renin cell progenitors invade the vessel wall and stimulate the concentric accumulation of smooth muscle cells leading to renal hypoperfusion and kidney injury. Given that the concentric hypertrophy is prevented by genetic killing of renin cells -with diphtheria toxin or B1 integrin deletion- it is clear that renin cells are directly responsible for the pathology. However, the mechanisms responsible remain to be elucidated. Given the new guidelines – lowering blood pressure percentiles- for blood pressure control and the extended use of RAS inhibitors in adults and children, it is imperative that we understand the pathogenesis of this silent, pervasive, and basically ignored vascular disease. This proposal has been designed to understand the mechanisms that maintain a healthy kidney vasculature without which a healthy kidney cannot exist, and how those mechanisms are altered when overall homeostasis is compromised.
Our overarching hypothesis is that regulation of progenitor endowment, cell fate, and maintenance of the differentiated arteriolar cells phenotypes are crucial for the acquisition and preservation of the architectural and functional integrity of the kidney. This hypothesis implies that early developmental events and cellular choices (such as epigenetic decisions in response to environmental cues throughout life) affect the physiological responses and the progression of kidney disease and/or repair mechanisms in the maturing and adult individual. As mentioned above, renin cell progenitors are crucial in the morphogenesis of the kidney vasculature. However, chronic relentless stimulation of renin cells leads to a severe, albeit silent, form of concentric hypertrophy of the intrarenal arteries and arterioles.
Because ACEi/ARBs are the most frequently used drugs in children to treat hypertension, and to slow the progression of CKD, it is imperative that we understand the serious, long-term consequences these drugs have on the renal vasculature in high-risk infants and children. Thus, we have focused the PCEN on this crucial and understudied topic of pediatric nephrology research. Two interrelated major projects have been designed to define the major mechanisms and the underlying events (transcriptional-epigenomic, signaling, and extracellular matrix interactions) that determine and amplify the progression of this severe renal arterial disease.
Projects
The following hypotheses will be tested:
Project 1. Under constant, unrestrained stimulation, renin cells suffer progressive and loci-specific changes in their chromatin responsible for concentric hypertrophy of the intrarenal arteries and arterioles. Renin cells acquire a dual embryonic-senescent phenotype driven by the constant activation of the cAMP/p300 pathway. In turn, the transformed renin cells via Notch activation induce the concentric vascular hypertrophy of the adjacent arteriolar and smooth muscle cells.
Project 2. Under a physiological threat to maintain the extracellular fluid volume and/or renal perfusion in infancy, the TGF-b/FGF-2 axis maintains the normal fate and function of renin and smooth muscle cells. When the balance between the RAS, TGF-b, and FGF-2 pathways is disrupted, cells programed for the renin phenotype integrate in a disorderly manner within the renal arterioles, precipitating the development of concentric arteriolar hypertrophy, poor renal perfusion, and kidney fibrosis.
