Thiele Technology Laboratory
Welcome to Dr. Thiele's Lab
The purpose of our research laboratory is to develop and test novel biomedical technology. This technology ultimately helps us take better care of both patients undergoing surgery and anesthesia, as well as those who are critically ill. Specific areas of interest for our group include the following:
Mitochondrial Near Infrared Spectroscopy
Our laboratory built and validated Mitochondrial Near Infrared Spectroscopy (NIRS) technology that is capable of measuring the oxidation state of cytochrome aa 3 (CYT OX ), the terminal component of the electron transport chain, in addition to tissue hemoglobin oxygenation (StO 2 ). Unlike StO 2 , which does not interrogate tissue directly, CYT OX measures whether or not tissues are adequately oxygenated, and correspondingly whether the electron transport chain functions as it should. Our interest now is to make this technology both smaller and more affordable, to the point that the hardware could be realistically utilized in an operating room or in an ICU environment.
Physiologic Waveform Analysis
We spent years analyzing photoplethysmographic, systemic arterial, central venous pressure, and pulmonary artery pressure waveforms. These efforts improve upon the dynamic measures of fluid responsiveness (e.g. pulse pressure variation), which are currently utilized by clinicians. Our current work focuses on the interaction between various waveforms, including central venous pressure and how these interactions can offer predictive value.
Wearables and Exercise Preconditioning
We study the amount of physical activity performed by pre-surgical patients and the role that this activity plays in complications after surgery. In our laboratory, we also expose animals to three weeks of treadmill training prior to undergoing surgery, in order to better understand the clinical impact of exercise training, as well as understand the underlying biological mechanism. We collaborate with our surgical and engineering school colleagues on a randomized pilot study for patients undergoing cancer surgery on a smart-phone based, heart-rate guided pre-surgical training program.
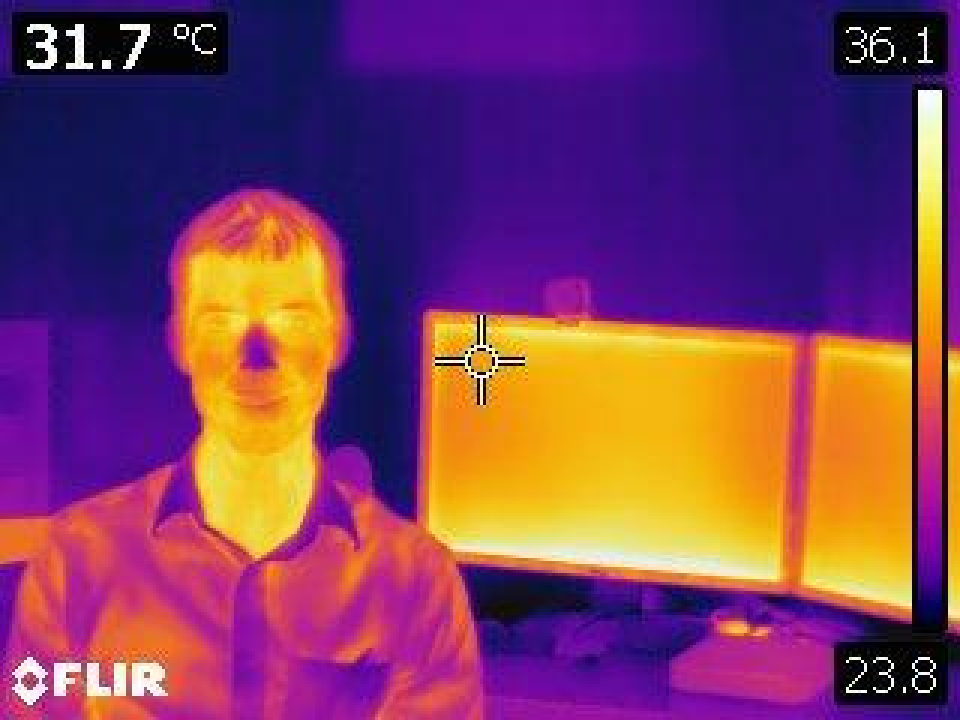
Thermography and Infrared Videography
Both cardiac pulsatility and airflow through the oropharynx can be detected using camera equipment with sensitivity in the infrared range of the electromagnetic spectrum. We are interested in using this equipment to develop technology to measure vital signs (respiratory rate, heart rate, and oxygen saturation) from camera systems that require no patient contact.
About Dr. Thiele's Lab

Robert Thiele, M.D.
Critical Care Anesthesia Division Chief
Dr. Robert H. Thiele, M.D., is a cardiac anesthesiologist and intensivist. Dr. Thiele’s undergraduate degree is in chemical engineering, and early in his anesthesiology residency Dr. Thiele recognized that anesthesiologists and intensivists were totally dependent upon technology in order to do their jobs safely and efficiently. This theme was noted in the now famous book “To Err Is Human: Building a Safer Health System,” published by the Institute of Medicine in 2000, in which anesthesia is specifically mentioned as an exemplary medical subspecialty.
An expert in the intersection of technology and patient safety, Dr. Thiele is regularly asked to review or write peer-reviewed manuscripts related to technology. In addition to his technology-focused research, Dr. Thiele runs the Critical Care Division in the Department of Anesthesiology, and is an at-large member of the Board of Directors for the University Physicians Group. A member of the APSF Committee on Technology, he was recently tasked with re-vamping the incident reporting system used by the UVA Health System.
“Anesthesiology is an example of a local, but complex, high-risk, dynamic patient care system in which there has been notably reduced error. Responding to rising malpractice premiums in the mid-1980s, anesthesiologists confronted the safety issues presented by the need for continuing vigilance during long operations but punctuated by the need for rapid problem evaluation and action. They were faced with a heterogeneity of design in anesthesia devices; fatigue and sleep deprivation; and competing institutional, professional, and patient care priorities. By a combination of technological advances (most notably the pulse oximeter), standardization of equipment, and changes in training, they were able to bring about major, sustained, widespread reduction in morbidity and mortality attributable to the administration of anesthesia.” –To Err Is Human: Building a Safer Health System, p. 164
While anesthesiology itself is now EXTREMELY safe (mortality rates are so low they are difficult to measure), 30-day mortality after surgery remains high, with approximately 200,000 Americans dying annually within 30 days of inpatient surgery. The anesthesiology community has done a phenomenal job of protecting patients in the operating room.
Future gains will come from optimizing the pre- and post-anesthesia care phases. Both of these will likely benefit from novel technological systems, including device-guided prehabilitation regimens to optimize pre-surgical fitness and risk-stratify patients. The following will also improve our current efforts: next-generation intraoperative and ICU monitoring systems to monitor tissue oxygenation and volume status/fluid responsiveness, continuous non-invasive monitoring systems in patients admitted to surgical floors, and the ability to remotely monitor patients after discharge from the hospital.
Active
NIH Trailblazer Award for New and Early Stage Investigators, Awarded by the NIBIB (2021-24) –$576,122, for a project entitled “Discrete Wavelength Frequency Domain Near Infrared Spectroscopy for Non-Invasive Measurement of Cytochrome Oxidation State,” (R21EB031780; P.O. Afrouz Azari Anderson).
Apple Investigator Initiated Award (2020) engineering support and equipment.
Awarded by Apple for project entitled “Exercise Preconditioning in Cancer Surgery.” Apple to provide watches as well as support to develop technology designed to encourage and monitor physical activity in surgical patients before and after their procedure(s), in addition to facilitating compliance monitoring and communication with the healthcare team.
Completed
Department of Surgery Research Award, Awarded by the Department of Surgery at the University of Virginia (2021) – $70,000, for project entitled “Correlation Between Heart Rate Variability and Pulse Amplitude Variation to Predict Fluid Responsiveness in Hemorrhagic Pig Model” along with Co-PI Dr. Mark Roeser (Pediatric Cardiac Surgeon). The purpose of this work is to develop a system for predicting the hemodynamic response to bleeding based on advanced analysis of arterial waveforms.
George A. Beller, M.D. Research Award, Awarded by the University of Virginia (2020) – $40,000, for project entitled “Regional Anticoagulation for Extracorporeal Membrane Oxygenation” along with Co-PI Dr. Mark
Roeser (Pediatric Cardiac Surgeon). The purpose of this work is to develop a system for anti-coagulating an ECMO circuit without requiring systemic anticoagulation, using a porcine
model.
Engineering in Medicine ,Awarded by the University of Virginia (2020) – approximately $75,000, for project entitled “Wearable Technology-Guided Exercise Preconditioning to Accelerate Return of Function After Cancer Surgery” along with Co-PIs Dr. Laura Barnes (Engineering) and Dr. Traci Hedrick (Medicine). The purpose of this work is to study the impact of wearable technology on VO 2 max in cancer patients presenting for surgery.
NIH Mentored Clinical Scientist Research Career Development Award (2016-2020) – $706,896.
Awarded by the NIGMS for project entitled “Effect of Sepsis on Cytochrome aa 3 Oxidation State.” (1K08GM115861-01A1; P.O. Sarah Dunsmore).
Society of Cardiovascular Anesthesiologists Starter Grant (2013-15) – $50,000.
Awarded by the SCA for project entitled “Comparison of Broad-Band Near-Infrared Spectroscopy-Derived Mitochondrial Oxidation State and Tissue Oxygen
Saturation for the Detection of Ischemia in Two Models of Rat Cerebral Ischemia.”
Ivy Foundation Biomedical Innovation Fund at the University of Virginia (2012-14) – $80,000, to develop a near infrared spectroscopy device to measure the oxidation state of cytochrome aa 3.

Hari Prasad Osuru, Ph.D.
Research Scientist
HO2B@virginia.edu
Dr. Osuru was born and raised in a small village in the South part of India. He received his Bachelor of Science in Biology with a minor in Microbiology from SV University. He completed his Masters of Science in Gene Technology, and Master of Philosophy in Biotechnology from Bharathidasan University. He received his Ph.D. in Biotechnology from SV. Institute of Medical Sciences, India.
Dr. Osuru’s research interests include identifying key molecular pathways associated with anesthesia and surgery that lead to changes in brain-organs function. Dr. Osuru’s current research focus in Dr. Robert Thiele’s Lab is studying Subcellular Energetics in Hypoxia, Inflammation, and Stress conditions, and studying Molecular Mechanisms of Physical Exercise in Sepsis. During his free time, he enjoys spending time with friends and family, cooking, gardening, watching historical documentaries, and listening to music.

Keita Ikeda, Ph.D.
Research Scientist
KI2D@uvahealth.org
Dr. Ikeda received his Bachelor of Science in Aerospace Engineering from the University of Southern California. He completed his Masters of Science in Aerospace Engineering with a minor in Mathematics at North Carolina State University. He received his Ph.D. in Biomedical Engineering at the University of North Carolina, Chapel Hill.
Dr. Ikeda’s current research focus in Dr. Robert Thiele’s Lab is studying Subcellular Energetics in Hypoxia, Inflammation, and Stress conditions and studying Molecular Mechanisms of Physical Exercise in Sepsis.
1. Knio ZO, Morales FL, Shah KP, Ondigi OK, Selinski CE, Baldeo CM, Zhuo DX, Bilchick KC, Mehta NK, Kwon Y, Breathett K, Thiele RH, Hulse MC, Mazimba S., “A systemic congestive index (systemic pulse pressure to central venous pressure ratio) predicts adverse outcomes in patients undergoing valvular heart surgery.” J Card Surg. 2022 Jul 17 [Epub ahead of print].
2. Ikeda K. Osuru HP. Thiele RH. “Intraoperative administration of isoflurane improves survival in rats exposed to caecal ligation and puncture.” British Journal of Anesthesia Open. June 2022 [Epub 100014].
3. Knio ZO, Thiele RH, Wright WZ, Mazimba S, Naik BI, Hulse MC. “A Novel Hemodynamic Index of Post-operative Right Heart Dysfunction Predicts Mortality in Cardiac Surgical Patients.” Semin Cardiothorac Vasc Anesth. [Epub ahead of print, March 25 2022].
4. Kane WJ, Hassinger TE, Chu DL, Myers EL, Charles AN, Hoang SC, Friel CM, Thiele RH, Davis EM, Hedrick TL. “Preoperative REM sleep is associated with complication development after colorectal surgery.” Surg Endosc. [Epub ahead of print, May 12, 2021].
5. Kane WJ, Hassinger TE, Myers EL, Chu DL, Charles AN, Hoang SC, Friel CM, Thiele RH, Hedrick TL. “Wearable technology and the association of perioperative activity level with 30-day readmission among patients undergoing major colorectal surgery.” Surg Endosc. [Epub ahead of print, March 29, 2021].
6. Osuru HP, Paila U, Ikeda K, Zuo Z, Thiele RH. “Anesthesia-Sepsis-Associated Alterations in Liver Gene Expression Profiles and Mitochondrial Oxidative Phosphorylation Complexes.” Front Med (Lausanne). 7: 581082, 2020.
7. Kwon HM. Ikeda K. Kim SH. Thiele RH. “Non-contact thermography-based respiratory rate monitoring in a post-anesthetic care unit. Journal of Clinical Monitoring and Computing” [Epub ahead of print September 25, 2020].
8. Hedrick TL, Hassinger TE, Myers E, Krebs ED, Chu D, Charles AN, Hoang SC, Friel CM, Thiele RH. “Wearable Technology in the Perioperative Period: Predicting Risk of Postoperative Complications in Patients Undergoing Elective Colorectal Surgery.” Dis Colon Rectum 63: 538, 2020.
9. Moon YJ. Bechtel AJ. Kim SH. Kim JW. Thiele RH. Blank RS. “Detection of intratracheal accumulation of thick secretions by using continuous monitoring of respiratory acoustic spectrum: A preliminary analysis.” Journal of Clinical Monitoring & Computing [Epub ahead of print Jul 20, 2019].
10. Thiele RH. Osuru HP. Paila U. Ikeda K. Zuo Z. “Impact of Inflammation on Brain Subcellular Energetics in Anesthetized Rats.” BMC Neuroscience 20: 34, 2019.
11. Park HS, Kim SH, Park YS, Thiele RH, Shin WJ, Hwang GS. “Respiratory Variations in Electrocardiographic R-Wave Amplitude during Acute Hypovolemia Induced by Inferior Vena Cava Clamping in Patients Undergoing Liver Transplantation.” J Clin Med 8: 717, 2019.
12. Kwon HM. Kim SH. Park HS. Park YS. Moon YJ. Kim JM. Thiele R. “Augmentation of Electrocardiographic QRS R-Amplitude Precedes Radiocontrast-Induced Hypotension during Mobile Computed Tomography Scanning.” J Clin Med 8: 4, 2019.
13. Thiele RH, Ikeda K, Osuru HP, Zuo Z. “Comparison of Broadband and Discrete Wavelength Near Infrared Spectroscopy Algorithms for the Detection of Cytochrome aa 3 Reduction.” Anesthesia & Analgesia 129: 1273, 2019
14. Thiele RH. Ikeda K. Bartz RR. Wang Y. Zuo Z. “Broadband Near Infrared Spectroscopy Can Detect Cyanide-Induced Cytochrome aa 3 Inhibition in Rats: A Proof of Concept Study.” Canadian Journal of Anesthesia 3: 376, 2017.
15. Olenczak JB. Murariu D. Ikeda K. Thiele RH. Campbell CA. “Tissue Monitoring with 3-wavelength Light Emitting Diode Based Near Infrared Spectroscopy.” Journal of Reconstructive Microsurgery 32: 712, 2016.
16. Ikeda K. Smith GA. Renehan J. Isbell JM. McMurry TL. Rosner MH. Thiele R. “Multi-Parameter Predictor of Fluid Responsiveness in Cardiac Surgical Patients Receiving Tidal Volumes Less Than 10 mL/kg.” Seminars in Cardiothoracic and Vascular Anesthesia 20: 188, 2016.
17. Colquhoun DA. Naden KB. Thiele RH. “Frequency Domain Analysis of Cerebral Near Infrared Spectroscopy Signals During Application of an Impedance Threshold Device in Spontaneously Ventilating Volunteers.” Journal of Clinical Monitoring and Computing 30: 389, 2016.
18. Thiele RH. Bartels K. Esper S. Ikeda K. Gan TJ. “Real-Time Doppler-Based Arterial Vascular Impedance and Peripheral Pressure-Flow Loops: A Pilot Study.” Journal of Cardiothoracic and Vascular Anesthesia 28: 36, 2014.
19. Bartels K. Thiele RH. Phillips-Bute B. Glower DD. Swaminathan M. Kisslow J. Mackensen GB. “Dynamic Indices of Mitral Valve Function Using Perioperative Three-Dimensional Transesophageal Echocardiography.” Journal of
Cardiothoracic and Vascular Anesthesia 28: 18, 2014.
20. Thiele RH. Colquhoun DA. Forkin KT. Durieux ME. “Assessment of the Agreement Between Photoplethysmographic and Arterial Waveform Respiratory
Variation in Patients Undergoing Spine Surgery.” Journal of Medical Engineering & Technology 37: 409, 2013.
21. Colquhoun DA. McMurry T. Dunn L. Thiele RH. “The Relationship Between the Area of Peripherally-Derived Pressure Volume Loops and Systemic Vascular
Resistance.” Journal of Clinical Monitoring & Computing 27: 689, 2013.
22. Kiehna EN. Huffmyer JL. Thiele RH. Scalzo DC. Nemergut EC. “Utilizing the Intrathoracic Pressure Regulator to Lower Intracranial Pressure in Patients with Altered Intracranial Elastance: A Pilot Study.” Journal of Neurosurgery 119: 756, 2013.
23. Colquhoun DA. Dunn LK. Thiele RH. “The Relationship Between Respiratory Variation in the Pulmonary Arterial Pressure Tracing and Intrathoracic Pressure Changes: A Pilot Study.” Journal of Medical Engineering & Technology 37: 252, 2013.
24. Colquhoun DA. Forkin K. Dunn LD. Bogdonoff DL. Durieux ME. Thiele RH. “Non-Invasive, Minute-to-Minute Estimates of Systemic Arterial Pressure and Pulse Pressure Variation Using Radial Artery Tonometry.” Journal of Medical Engineering & Technology 37: 197, 2013.
25. Thiele RH. Knipper E. Dunn LK. Nemergut EC. “Auditory Stimuli as a Contributor to Consciousness While Under General Anesthesia.” Medical Hypothesis 80: 568–72, 2013.
26. Thiele RH. Colquhoun DA. Tucker-Schwartz J. Gillies GT. Durieux ME. “Radial-Femoral Concordance in Time and Frequency Domain-Based Estimates of Arterial Respiratory Variation.” Journal of Clinical Monitoring and Computing 26:
393, 2012.
27. Thiele RH. Colquhoun DA. Blum FE. Durieux ME. “Ability of Anesthesia Providers to Visually Estimate Systolic Pressure Variability Using the ‘Eyeball’ Technique.”
Anesthesia & Analgesia 115: 176, 2012.
28. Colquhoun DA. Tucker-Schwartz J. Durieux ME. Thiele RH. “Non-Invasive Estimation of Jugular Venous Oxygen Saturation: A Comparison Between Near Infrared Spectroscopy and Transcutaneous Venous Oximetry.” Journal of Clinical Monitoring and Computing 26: 91, 2012.
29. Thiele RH. Colquhoun DA. Gillies GT. Tiouririne MT. “Manipulation of Hyperbaric Lidocaine Using a Weak Magnetic Field: A Pilot Study.” Anesthesia & Analgesia
114: 1365, 2012.
30. Colquhoun DA. Forkin KT. Durieux ME. Thiele RH. “Ability of the Masimo Pulse Co-Oximeter To Detect Changes in Hemoglobin.” Journal of Clinical Monitoring and Computing 1: 26, 69, 2012.
31. Thiele RH. Colquhoun DA. Patrie J. Nie SH. Huffmyer JL. “Relationship Between Plethysmographic Waveform Changes and Hemodynamic Variables in Anesthetized, Mechanically-Ventilated Patients Undergoing Continuous Cardiac Output Monitoring.” Journal of Cardiothoracic and Vascular Anesthesia 25: 1044, 2011.
32. Thiele RH. Tucker-Schwartz J. Lu, Y. Gillies, GT. Durieux, ME. “Transcutaneous Regional Venous Oximetry: A Feasibility Study.” Anesthesia & Analgesia 112: 1353, 2011.
33. Huffmyer JL, Groves DS, DeSouza DG, Littlewood KE, Thiele RH, Nemergut EC. “The Effect of the Intrathoracic Pressure Regulator on Hemodynamics and Cardiac Output.” Shock 35: 114, 2011.
1. Michard F. Thiele RH. Saugel B. Joosten A. Flick M. Khanna AK. “Wireless Wearables for Postoperative Surveillance on Surgical Wards: A Survey of 1158 anesthesiologists in Western Europe and the USA.” Accepted to BMJ Open on 1/12/22.
2. Michard F. Thiele RH. Le Guen M. “One small wearable, one giant leap for patient safety?” J Clin Mon Computing Oct 12 2021 [Epub ahead of print].
3. Thiele RH. Shaw AD. Bartels K. Brown CH. Grocott H. Heringlake M. Gan TJ. Miller TE. McEvoy MD. “American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) Joint Consensus Statement on The Role of Neuromonitoring in Perioperative Outcomes: Cerebral Near-Infrared Spectroscopy.” Anesthesia & Analgesia 131: 1444, 2020.
4. Saugel B. Thiele RH. Hapfelmeier A. “Cannesson M. Technological Assessment and Objective Evaluation of Minimally-Invasive and Non-Invasive Cardiac Output
Monitoring Systems.” Anesthesiology 133: 921, 2020.
5. Chan MTV, Hedrick TL, Egan TD, García PS, Koch S, Purdon PL, Ramsay MA, Miller TE, McEvoy MD, Gan TJ; Perioperative Quality Initiative (POQI) 6 Workgroup. “American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on the Role of Neuromonitoring in Perioperative Outcomes: Electroencephalography.” Anesth Analg 130: 1278,
2020.
6. Roach JK. Thiele RH. “Perioperative Blood Pressure Monitoring.” Accepted to Best Practice & Research: Clinical Anaesthesiology 33: 127, 2019.
7. Thiele RH. “Aligning Health Care and Academia: The Clinician Innovator.” Academic Medicine 93: 960, 2018.
8. Thiele RH. Letter to the Editor: “Organ dysfunction after surgery in patients treated with individualized or standard blood pressure management.” Journal of American Medical Association 319: 720, 2018.
9. Thiele RH. Editorial: “Venous Circulation and the Invisible Hand.” Canadian Journal of Anesthesia 65: 231, 2018.
10. Thiele RH. “The Anesthesiologist’s Dream: ‘Wireless’ Vital Sign Monitoring?” Anesthesia & Analgesia 125: 724, 2017.
11. Thiele RH. “Subcellular Energetics and Metabolism: Potential Therapeutic Applications.” Anesthesia & Analgesia 124: 1872, 2017.
12. Thiele RH. “Subcellular Energetics and Metabolism: A Cross-Species Framework.” Anesthesia & Analgesia 124: 1857, 2017.
13. Grocott HP. Thiele RH. “Brain Tissue Oximetry: what are we really measuring?” Anesthesia & Analgesia 124: 2091, 2017.
14. Raphael J, Regali LA, Thiele RH. “Hemodynamic monitoring in thoracic surgical patients.” Curr Opin Anaesthesiol. 2017 Jan 3 [Epub ahead of print].
15. Bartels K. Esper SA. Thiele RH. “Blood Pressure Monitoring for the Anesthesiologist: A Practical Review.” Anesthesia & Analgesia 122: 1866, 2016
16. Thiele RH. “Bulldozers, Backhoes, and Blood Pressure.” Anesthesia & Analgesia 121: 1417, 2015.
17. Bartels K. Thiele RH. “Advances in Photoplethysmography: Beyond Arterial Oxygen Saturation.” Can J Anaesth 62: 1313, 2015.
18. Thiele RH. McMurry T. “Data Agnosticism and Implications on Method Comparison Studies.” Anesthesia & Analgesia 121: 264, 2015.
19. Thiele RH. Bartels K. Gan TJ. “Inter-Device Differences in Monitoring for Goal-Directed Fluid Therapy.” Canadian Journal of Anesthesia 62: 169, 2015.
20. Thiele RH. Bartels K. Gan TJ. “Cardiac Output Monitoring: A Contemporary Assessment and Review.” Critical Care Medicine 43: 177, 2015.
21. Thiele RH. Gan TJ. “Preface: Hemodynamic Monitoring Devices. Best Practice & Research” Clinical Anaesthesiology 28: 305, 2014
22. Bartels K. Thiele RH. Gan TJ. “Rational fluid management in today’s ICU practice.” Critical Care 17S1: S6, 2013.
23. Colquitt RB. Colquhoun DA. Thiele RH. “In silico modeling of physiologic systems. Best Practice & Research” Clinical Anaesthesiology 25: 499, 2011.
24. Thiele RH. Durieux ME. “Arterial Waveform Analysis for the Anesthesiologist – Past, Present, and Future Concepts.” Anesthesia & Analgesia 113: 766, 2011.
25. Thiele RH. Nemergut EC. Lynch CL. “The Clinical Implications of Isolated Alpha 1 Adrenergic Stimulation.” Anesthesia & Analgesia 113: 297, 2011.
26. Thiele RH. Nemergut EC. Lynch CL. “The Physiologic Implications of Isolated Alpha 1 Adrenergic Stimulation.” Anesthesia & Analgesia 113: 284, 2011.
