Susanna R. Keller, MD
 PRIMARY APPOINTMENT:
PRIMARY APPOINTMENT:
- Associate Professor of Medicine, Endocrinology and Metabolism (primary)
- Associate Professor of Cell Biology (secondary)
CONTACT:
Aurbach Building Room 2322
450 Ray C Hunt Drive
Charlottesville VA 22903
Email: srk4b@virginia.edu
RESEARCH AREAS:
- Insulin signaling and regulation of nutrient metabolism and energy homeostasis
- Metabolic disease-associated cardiovascular complications
- Hormone-regulated membrane trafficking and relevance to whole-body physiology
EDUCATION AND TRAINING
- Graduate: M.D. (Dr. Med.) University of Zurich, Switzerland
- Research Fellow: Metabolic Unit, University of Zurich, Switzerland
- Postdoctoral Fellow: Dartmouth Medical School, Biochemistry, Hanover NH
FUNDED PROJECTS
- NIH/NHLBI R01 HL128189 (1/1/2021 – 12/31/2024)
RESEARCH SUMMARY
A major interest of the Keller laboratory has been to investigate insulin signaling and the regulation of the trafficking of membrane proteins, the glucose transporter GLUT4, and the insulin-regulated aminopeptidase IRAP in primary adipocytes and skeletal muscles, and to study how insulin, by promoting cell surface localization of GLUT4 and IRAP in adipocytes and muscles, affects whole-body nutrient metabolism and energy homeostasis. Recent research examined the roles of two Rab GTPase activating proteins (Rab GAPs), AS160 (Tbc1d4) and Tbc1d1, in the regulation of glucose uptake in primary adipocytes and skeletal muscles and the control of whole-body glucose, lipid, and energy homeostasis using knockout mice and primary adipocytes and skeletal muscles isolated from these.
Recently, Dr. Keller took the lead of a collaborative research project investigating molecular mechanisms by which the renal angiotensin receptor type 2 (AT2R) regulates sodium excretion and blood pressure. We use rat models of hypertension and proximal tubule-specific AT2R knockout mice to delineate pathways controlling AT2R trafficking to the apical surface of renal proximal tubule cells, identify AT2R signaling intermediates that promote sodium excretion, and uncover defects in AT2R trafficking and signaling that contribute to the development of hypertension.
In long-term research collaborations with other investigators at the University of Virginia, Dr. Keller is involved in evaluating the effects of genetic background on metabolic responses to various diets, elucidating the role of adenosine receptors in metabolic homeostasis, identifying metabolic defects preceding hypertension-induced left ventricular hypertrophy and heart failure, and investigating the role of metabolic disease in the development of atherosclerosis. The research in the Keller laboratory is relevant to major metabolic diseases (metabolic syndrome, obesity, and diabetes) and associated cardiovascular complications.
Collaborators at the University of Virginia
- David Brautigan PhD, Professor Emeritus of Pharmacology
- Robert Carey MD MCAP, Professor and Dean Emeritus, Department of Medicine-Division of Endocrinology and Metabolism
- Mete Civelek PhD, Associate Professor of Biomedical Engineering and Resident Faculty in the Department of Genome Sciences
- Heather Ferris, MD PhD, Assistant Professor of Medicine, Endocrinology and Metabolism
- John Gildea PhD, Research Scientist, Department of Pathology
- Thurl Harris PhD, Associate Professor of Pharmacology
- Sibylle Kranz PhD RDN, Associate Professor of Kinesiology
- Bijoy Kundu PhD, Associate Professor of Radiology and Medical Imaging
- Gary Owens PhD, Professor of Cardiovascular Research, Department of Molecular Physiology and Biological Physics
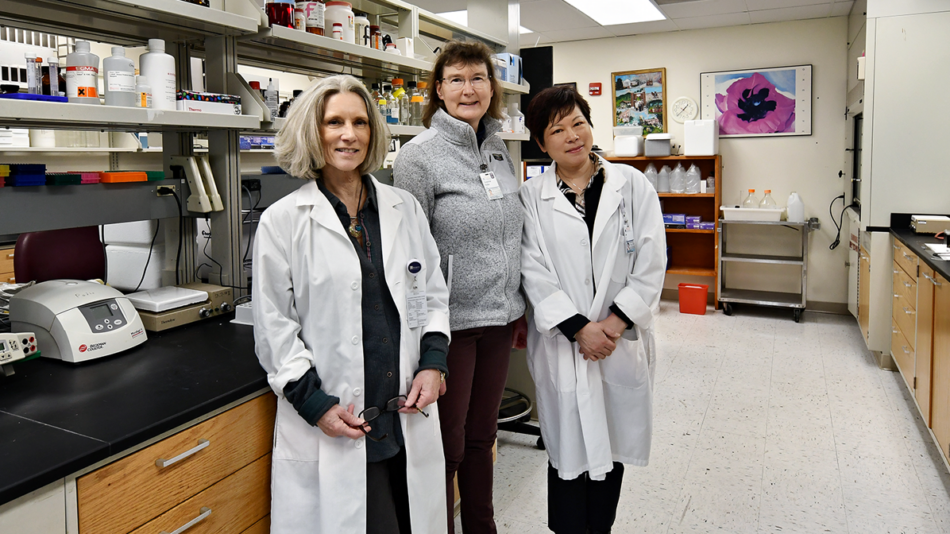
KELLER LAB MEMBERS

From lerft: Nancy Howell, Susanna Keller, and Jie Li
 Laboratory Specialist Advanced
Laboratory Specialist Advanced
Nancy Howell was born in Brooklyn, N.Y., and raised in Sparta, N.J.
She attended Mary Baldwin College (now the University) and graduated in 1982. In 1983, Nancy started working for Dr. Robert M. Carey. She worked for Dr. Carey for 41 years until his retirement last year, when she began working with Dr. Keller as she continued the lab’s research.
Nancy will retire in 2025 with 42 years of service in the same lab, working with basic science NIH grants. She lives with her husband, three dogs, and two cats in Albemarle County.
 Research Scientist
Research Scientist