Methods and Resources
Methods and Resources
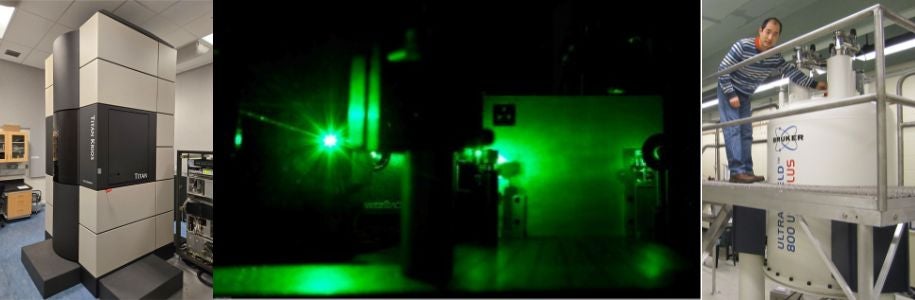
The UVa Molecular Electron Microscopy Core laboratory enables cryoEM and tomography for high-resolution imaging and three-dimensional reconstruction. A state-of-the-art Titan Krios has been installed in 2012 and has been equipped with a Falcon II direct detector in 2014. A Glacios microscope was installed in 2022 and an Aquilos-2 FIB-SEM will be installed in the Spring 2024. FEI Spirit and F20 electron microscopes are also part of the core.
Core Director: Michael Purdy
 The biomolecular nuclear magnetic resonance facility at UVa is home to one 800, three 600, and two 500 MHz spectrometers, which are equipped with triple-resonance cryoprobes. A 500 MHz solid-state NMR spectrometer with a heteronuclear MAS-probe is available for solids experiments.
The biomolecular nuclear magnetic resonance facility at UVa is home to one 800, three 600, and two 500 MHz spectrometers, which are equipped with triple-resonance cryoprobes. A 500 MHz solid-state NMR spectrometer with a heteronuclear MAS-probe is available for solids experiments.
More: https://med.virginia.edu/biomolecular-magnetic-resonance-facility/
Core Director: Jeff Ellena
EPR equipment includes two X-band cw spectrometers, a pulse X-band machine capable for DEER, and a pulse Q band spectrometer.
Robotics for membrane protein crystallization is available. These instruments can set up batch or vapor diffusion experiments and the protein crystals can be visualized and docmented using polarized visible light or UV fluorescence microscopes at 4 and 17 degrees Celsius.
Research Faculty: Michael Purdy
The Center houses culture facilities for large-scale expression in bacterial, yeast, insect, and mammalian cell systems. Protein purifications and characterizations are handled by individual labs and collaboratively as needed.
Research Faculty: Mark Daniels
Confocal, TIRF, lifetime, and laser microscopes for cell biological research and single molecule detection are available to Center researchers.
Research Faculty: Volker Kiessling, Kandice Levental
For University and School of Medicine-wide Cores, see:
W. M. Keck Center for Cellular Imaging
Director: Ammasi Periassamy
Director: Sijie Hao
Synchrotron beam time is available at the Argonne National Lab Advanced Photon Source.
Research Faculty: Michael Purdy
