Equipment
Click here for a full equipment list across all School of Medicine Research Cores
Benchtop Analyzers

- 3 lasers – blue (488nm – 50mW), violet (405nm – 100mW) and red (640nm – 80mW)
- 2 scattering channels
- up to 38 fluorescence detectors
Additional Resources
Optimized Multicolor Immunofluorescence Panel (OMIP) is a special peer-reviewed Cytometry Part A publication type that reports on newly designed and optimized multicolor panels for flow cytometry.
Click here for a panel design grid to help you design antibody panels.
Click here to evaluate and optimize your panel design using the Cytek Full Spectrum Viewer.

- 5 lasers – blue (488nm – 50mW), violet (405nm – 100mW), yellow-green (561nm – 50mW), red (640nm – 80mW) and UV (355nm – 20mW)
- 3 scattering channels
- up to 64 fluorescence detectors
- Autosampler – 96 well plate
Additional Resources
Optimized Multicolor Immunofluorescence Panel (OMIP) is a special peer-reviewed Cytometry Part A publication type that reports on newly designed and optimized multicolor panels for flow cytometry.
Click here for a panel design grid to help design your antibody panels.
Click here to evaluate and optimize your panel design using the Cytek Full Spectrum Viewer.

- 4 laser lines – blue (488nm), red (640nm), violet (405nm), and yellow-green (561nm)
- 16 parameters (FSC, SSC and 14 fluorescent parameters)
- Incorporated high-resolution, high-speed brightfield microscope capable of capturing up to 6,000 images/second
- Autosampler – 96-well, 384-well, and deep-well plates
Click here for Attune CytPix Optical Configuration.

- 4 laser lines – blue (488nm), red (640nm), violet (405nm), and yellow-green (561nm)
- 16 parameters (FSC, SSC and 14 fluorescent parameters)
- Autosampler – 96-well, 384-well, and deep-well plates; 1.5 mL and 2 mL microcentrifuge tube rack (up to 24 per rack); Passive cooling available for 96-well U-bottom plates and microcentrifuge tube racks
Click here for ATTUNE NxT Optical Configuration.

- 4 laser lines (488nm, 405nm, 561nm, and 640nm)
- 18 parameters; 2 scatter, 3 colors off the blue laser, 5 colors off the yellow-green laser, 3 colors off the red and 6 off the violet.
Click here to access the optical layout.
Plan your panel for the LSRFortessa here.
All individuals who desire training must first complete the DIVA 6.0 Online Tutorial (even those with previous DIVA experience) as the training will assume a basic knowledge of the software.
See Scheduling for information on scheduling time on this instrument.

A flexible analyzer based on the principles of flow cytometry enables you to multiplex (simultaneously measure) up to 50 analytes in a single microplate well, using very small sample volumes. The system delivers fast and cost-effective bioassay results on many assay formats including nucleic acid assays, receptor-ligand assays, immunoassays and enzymatic assays.
-
- For a list of companies who supply reagent kits for use on the Luminex platform click here.
- For a list of publications for this technology click here.
- For a list of applications and updates for this technology click here.
- For a link to general protocols try this xMAP Luminex Cook Book
This instrument is located in Pinn Hall, Rm 2011. 434-243-2711
Cell Sorters

Like the Aurora full spectral cytometers, the Aurora CS provides the benefits of full spectral cytometry. Easily transition of already optimized high-dimensional fluorescent panels directly from existing Aurora Spectral Cytometers to the Aurora CS Cell Sorter. Ability to perform high purity cell sorting on complex cell subsets and recover high-dimensional analytical data above 40+ fluorescent markers, maximizing data generated on valuable limited samples.
- Capable of detecting up to 64 fluorescent parameters and 3 scatter parameters
- 6-way sorting 1.5ml tubes
- 4-way into 5 ml tubes
- 96 well plate sorting
- BSC Class A2 level II biosafety cabinet for sorting BSL-2 biohazardous samples

- 4 laser lines – violet (405nm), blue (488nm), yellow-green (561nm) and red (638nm)
- 14 parameters – forward & side scatter and 12 fluorescent detectors
- Two and four-way sorting into tubes as well as 6-, 12-, 24-, 48-, 96-, and 384-well plates.
- BSC Class A2 level II biosafety cabinet for sorting BSL-2 biohazardous samples.
- Automated setup

- Isolation of cell populations labelled with 50nm superparamagnetic particles
- Choice of manually labeling cells offline or having the autoMACS Pro label them for you as part of the isolation protocol.
- Several different automated protocols based on:
- Density of the antigen
- Frequency of the population
- Negative or positive selection
- Isolation directly from whole blood
AutoMACS Pro Time Per Sample Calculator
Mass Cytometer

The CyTOF XT Mass Cytometer simultaneously resolves multiple parameters with minimal signal overlap, providing unprecedented insights into the functional and phenotypic complexity of biological systems at the single cell level.
The CyTOF XT analyzes cells labeled with a panel of reagents conjugated to heavy metal isotopes using state-of-the art time of flight mass cytometry technology.
With over 100 detection channels and over 30 isotopic metal probes, CyTOF XT simplifies panel design and unlocks the functional and phenotypic complexity of biological systems at the single cell level.
Mass cytometry experiments routinely measure dozens of functional and phenotypic parameters in millions of single cells, output as .txt and .fcs files. Analysis of such high dimensional datasets is performed using a tool kit that includes not only traditional histograms and bivariate plots, but also novel clustering algorithms, neural networks, and dimensionality reduction methods.
CyTOF data analysis – High-Dimensional Single Cell Analysis
Consult the facility for metal combinations, appropriate controls, and other considerations prior to designing your experiment.
Located in Pinn Hall, Room 2013; 434-243-7682.
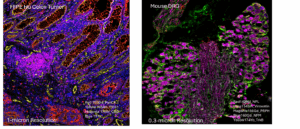
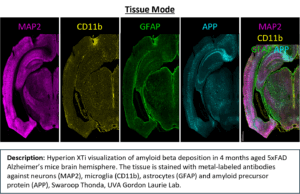
The Hyperion XTi Imaging Mass Cytometer™ combines proven CyTOF® mass-cytometry technology with high-resolution tissue imaging to enable simultaneous detection of 40 or more protein and RNA markers from a single FFPE or frozen section. Metal-tagged antibodies replace fluorophores, eliminating autofluorescence and spectral overlap for quantitative, multiplex spatial analysis at subcellular resolution.
Key Specifications:
- Multiplexing: 40 + markers simultaneously
- Resolution: 0.3 – 1 µm (Cell Mode), 5 µm (Tissue Mode)
- Throughput: 15 – 40 slides per day depending on mode and resolution
- Slide Capacity: 40-slide carousel for unattended runs (up to 72 hours continuous acquisition)
Acquisition Speed: ≈ 1.5 hours per mm²
Imaging Modes:
- Preview Mode – rapid whole-slide overview (1 µm resolution, 25 µm spacing)
- Cell Mode – high-resolution ROIs (0.3 – 1 µm subcellular detail)
- Tissue Mode – large-area scans (5 µm resolution)
Compatible Samples: FFPE and fresh-frozen tissue
Data Integration: exports to MCD Viewer, QuPath, CellProfiler, OMIQ, and other spatial analysis platforms
Associated Services:
- CyTOF XT antibody bank and metal conjugation support via the Flow Cytometry Core
- IMC slide preparation and staining through the Spatial Biology Core
Imaging Flow Cytometer

ImageStreamX Imaging Flow Cytometer
By combining the speed, sensitivity, and phenotyping capabilities of flow cytometry with the detailed imagery and functional insight of microscopy, the ImageStreamX overcomes the limitations of both techniques and opens the door to a wide range of applications, including:
-
Tracking protein translocation from cytoplasm to nucleus
-
Quantifying capping and co-capping reactions
-
Separating cells based on unique morphologic criteria
-
Studying cell–cell interactions
-
Differentiating cell states such as late apoptosis and necrosis
-
Examining cell signaling, co-localization, shape change, and internalization
You can define and quantify cell subpopulations not only by signal intensity, but also by the location of fluorescent probes on, in, or between cells—even in rare sub-populations or highly heterogeneous samples. Morphologic and nuclear features can also be used to characterize cell populations.
Instrument Features
-
Cells are illuminated with brightfield and 405 nm, 488 nm, 560 nm, and 658 nm lasers
-
Produces up to 12 high-resolution images (brightfield, darkfield, and up to 10 fluorescence channels) for each cell
-
Acquisition rates up to 5,000 cells per second
-
IDEAS software provides pre-defined fluorescence and morphologic parameters for quantitative, multi-parameter analyses
Extended Depth of Field (EDF)
Applications requiring enumeration of intracellular or intranuclear spots, such as fluorescence in situ hybridization (FISH), benefit from the EDF upgrade—a specialized combination of optics and image processing algorithms that project all structures within the cell into one crisp, focused image.
-
Enables accurate spot detection and counting throughout the cell
-
Maintains speed and sensitivity with no loss of resolution
Features and Upgrades
-
Mark II Upgrade
-
Real-time compensation, plotting, and gating
-
Gated events can be used as data storage criteria
-
Accepts sample volumes from 20–200 μL
-
95% sample utilization with new fluidic system
-
Redesigned, intuitive user interface
-
-
MultiMag Option
-
Provides 20X, 40X, and 60X objectives for greater flexibility
-
60X: higher magnification for yeast, bacteria, and fine cellular detail
-
20X: larger field of view for epithelial and large cells
-
-
96-Well Plate AutoSampler
-
Enables automated, unattended sample loading from multiwell plates
-
Ideal for dose–response and time course studies
-
Allows imaging of large numbers of cells from each sample
-
For more details:
Please refer to iLab for pricing.
Please refer to iLab for pricing
