Structure, dynamics, and function of rafts in cell membranes
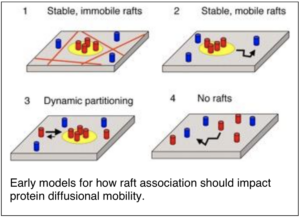
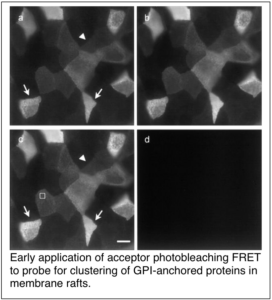
The proposal that cell membranes contain functional microdomains known as lipid rafts led to enormous interest about the structure and dynamics of these domains in live cell membranes. As a postdoctoral fellow I carried out some of the earliest studies using biophysical approaches such as FRET (Förster resonance energy transfer) and FRAP (fluorescence recovery after photobleaching) to test fundamental predictions of the lipid raft model. Our findings suggested that if lipid rafts exist in cells, they must be small and dynamic.
Even today, many unanswered questions still remain about rafts and their physiological functions. We have focused our studies on specialized rafts generated upon binding of bacterial toxins such as cholera toxin to their putative raft-associated glycolipid receptors on the cell surface. These studies led to observations that pointed us to the unexpected discovery of an entirely new mechanism of membrane bending in endocytosis, discussed in more detail below. In collaboration with the Sanders lab at Vanderbilt School of Medicine, we are also testing the long-standing hypothesis that rafts play a role in Alzheimer’s disease.
In addition to probing the functional roles of rafts in cells, our group is currently developing new approaches to chemically manipulate rafts by carrying out high throughput screening of giant plasma membrane vesicles (GPMVs). This commonly used membrane model captures key properties of rafts in native membrane environments and also enables direct visualization of co-existing raft and non-raft domains, making it an excellent candidate for screening purposes. To facilitate these efforts, we have developed software that can be used to perform high content image analysis. A major goal of these studies is to develop pharmacological tools that can be used to manipulate rafts without disrupting cellular cholesterol or glycosphingolipids, the most commonly used approaches in the field. These efforts may also uncover lead compounds that ultimately led to the development of new strategies to therapeutically target raft-related diseases in the clinic.
Recent publications
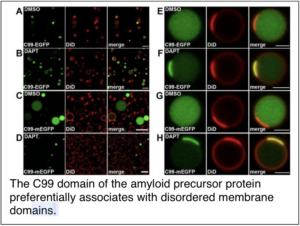
Capone, R., A. Tiwari, A. Hadziselimovic, Y. Peskova, J. M. Hutchison, C. R. Sanders and A. K. Kenworthy (2021) The C99 domain of the amyloid precursor protein resides in the disordered membrane phase. J Biol Chem. 2021:100652
Kabbani, A. M., K. Raghunathan, W. I. Lencer, A. K. Kenworthy, and C. V. Kelly (2020) Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin. PNAS 117(26): 14978-14986. PMCID: PMC7334530
Marinko, J. T., A. K. Kenworthy, and C. R. Sanders (2020) Peripheral Myelin Protein 22 preferentially partitions into ordered phase membrane domains. PNAS 117 (25): 14168-14177. doi: 10.1073/pnas.2000508117. PMCID: PMC7322011
Kenworthy, A. K. (2020) Choosing who can ride the raft. Nat Rev Mol Cell Biol 21: 566–567
Raghunathan, K. and A. K. Kenworthy (2018) Dynamic pattern generation in cell membranes: current insights into membrane organization. Biochim Biophys Acta 1860 (10): 2018-2031. PMCID: PMC6234104
Raghunathan, K.*, Foegeding N. J.*, Campbell, A. M., Cover, T. L., Ohi, M. D., andA. K. Kenworthy( 2018) Determinants of raft partitioning of the Helicobacter pylori pore-forming toxin VacA. Infect Immun.86(5). pii: e00872-17. doi: 10.1128/IAI.00872-17.PMCID: PMC5913846. *equal contributions
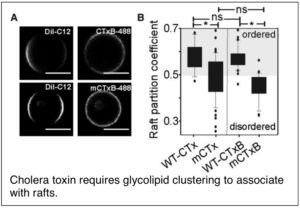
Raghunathan, K., T. Wong, D. Chinnapen, W.I. Lencer, M.G. Jobling,A. K. Kenworthy (2016) Glycolipid crosslinking is required for cholera toxin to target and stabilize ordered domains. Biophys J 111(12): 2547-2550.PMCID: PMC5192692
Schlebach, J. P., Barrett, P., Day, C. A., Kim, J. H., Kenworthy, A. K. and C. R. Sanders (2016) Topologically diverse human membrane proteins partition to liquid-disordered domains in phase-separated lipid vesicles. Biochemistry 55(7): 985-8. PMCID:PMC4766968
Day, C. A. and A. K. Kenworthy (2015) Functions of cholera toxin B-subunit as a raft cross-linker. Essays in Biochemistry 57:135-45. PMCID: PMC4346142
Day, C. A. and A. K. Kenworthy (2012) Mechanisms underlying the confined diffusion of cholera toxin B-subunit in intact cell membranes. PLoS ONE 7(4): e34923. PMCID: PMC3325267
Chinnapen, D., W. Hsieh, Y. t. Welscher, D. Saslowsky, L. Kaoutzani, E. Brandsma, L. D’Auria, H. Park, J. Wagner, K. Drake, M. Kang, T. Benjamin, M. Ullman, C. Costello, A. K. Kenworthy, B. T, R. Massol and W. I. Lencer (2012) Lipid-sorting by ceramide structure from plasma membrane to ER for the cholera toxin receptor ganglioside GM1. Dev. Cell 23: 573-86. PMCID: PMC3443397
