Clathrin-independent endocytosis
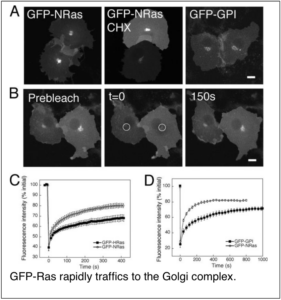
Localization to the correct subcellular compartment is key for proteins and lipids to function correctly in cells. Fluorescence microscopy offers direct insight into where proteins are localized intracellularly, and also often suggests potential mechanisms by which they are trafficked to and from their site of function. By visualizing proteins in living cells, we discovered two novel intracellular trafficking mechanisms. In the first case, we discovered that Ras, a key signaling protein often mutated in cancer, undergoes cycling by a combination of vesicular and non-vesicular transport mechanisms between the cell surface and Golgi complex. These observations helped to define a new paradigm for how palmitoylated proteins traffic within cells which has subsequently become generally accepted in the field.
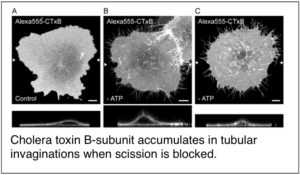
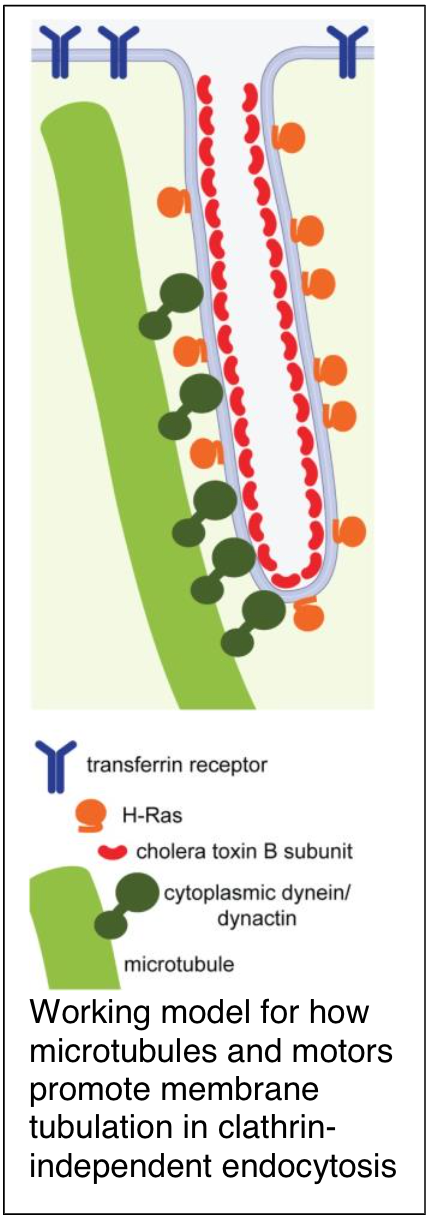
More recently, we have focused our efforts on a set of poorly understood trafficking pathways that mediate endocytosis in the absence of clathrin. We defined an entirely new mechanism for generating membrane curvature during clathrin-independent endocytosis in which microtubules, dynein, and dynactin provide a driving force for membrane tubulation. Concurrent collaborative studies with the Johannes and Bassereau groups suggest that microtubules and motors may work cooperatively with cellular machinery such as the BAR domain protein endophilin A2 to facilitate membrane scission as well. These findings set the stage for the identification of a new scission mechanism termed friction-driven scission. Experiments are currently underway to further probe the mechanisms by which microtubules and motors participate in CIE.
Recent publications
Simunovic, M., J.-B. Manneville, H. F. Renard, E. Evergren, K. Raghunathan, A. K. Kenworthy, G. A. Voth, J. Prost, H. T. McMahon, L. Johannes, P. Bassereau, A. Callan-Jones. (2017) Friction mediates scission of tubular membranes scaffolded by BAR proteins. Cell 170(1): 172-184. PMCID: PMC5576516
Chandrasekaran, R., A. K. Kenworthy, and D. B. Lacy (2016) Clostridium difficile Toxin A undergoes clathrin-independent, PACSIN2-dependent endocytosis. PLoS Pathog 12(12): e1006070. doi:10.1371/journal.ppat.1006070. PMCID: PMC5152916
Renard, H. F., M. Simunovic, J. Lemiere, E. Boucrot, M. D. Garcia-Castillo, S. Arumugam, V. Chambon, C. Lamaze, C. Wunder, A. K. Kenworthy, A. A. Schmidt, H. T. McMahon, C. Sykes, P. Bassereau and L. Johannes (2015) Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 517(7535): 493-6. PMCID: PMC4342003
Day, C. A., N. W. Baetz*, C. A. Copeland, L. J. Kraft, B. Han, A. Tiwari, K. R. Drake, H.De Luca, D. J. Chinnapen, M. W. Davidson, R. K. Holmes, M. G. Jobling, T. A. Schroer, W. I. Lencer and A. K. Kenworthy (2015) Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis. Traffic 16(6): 572-90. PMCID: PMC4440230
Goodwin, J. S., K. R. Drake, C. Rogers, L. Wright, J. Lippincott-Schwartz, M. R. Philips and A. K. Kenworthy (2005) Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol 170(2): 261-72.
