Research
The condensed protein states for gene regulation in cancer
A fundamental strategy cell uses for spatiotemporal control of biochemistry is forming biomolecular condensates often through liquid-liquid phase separation. These condensates are assembled via weak and multivalent interactions and often involve intrinsically disordered regions (IDRs) of proteins. Nuclear proteins are especially enriched with extensive IDRs and have close interactions with DNAs and RNAs, natural polymers that can act as nucleation sites for condensation and integrated regulation. These make nuclear phase separation particularly fascinating, but we are merely beginning to understand how it is regulated and how its dysregulation causes cancer.
We aim to address these fundamental questions:
• How do nuclear condensates regulate biology through gene regulation?
• How does condensate dysregulation cause cancer and other human diseases?
• How are condensates regulated?
• How can we target condensates for basic studies and disease intervention?
Biophysical properties of gene-regulatory condensates regulate cancer
My research group previously identified AKAP95, a nuclear zinc-finger protein, as a novel factor that integrates regulation of transcription and RNA splicing. We later generated an Akap95 knockout mouse model and found that AKAP95 is dispensable for normal cell and animal physiology but important for tumorigenesis through promoting cell proliferation and overcoming the senescence barrier to cancer, thus making AKAP95 a good target for cancer treatment. We are currently studying how AKAP95 inhibition may affect tumorigenesis in more physiologically relevant cancer models. Our preliminary data suggest that AKAP95 loss significantly suppresses leukemogenesis.
In pursuing the fundamental properties of AKAP95 that underlie its activities in splice and cancer regulation, our initial observation of purified AKAP95 displaying milk-like emulsion led to our discovery that AKAP95 undergoes liquid-liquid phase separation via its intrinsically disordered region. Through mutagenesis and replacement of the disordered region, we demonstrated a crucial functional role of AKAP95 phase separation in its activities in regulating splicing and tumorigenesis. Most interestingly, we found that a mutation that solidifies AKAP95 condensates significantly impairs its activity in regulating splicing and tumorigenesis. This is the first study demonstrating an important role of condensate biophysical properties in gene regulation and cancer (Fig. 1), and raise a novel possibility of cancer treatment by either disrupting or hardening cancer-driving condensates (Fig. 1) (Li, et al., Nat Cell Biol, 2020). The pharmacological feasibility was later shown (by another lab) by a condensate-hardening drug that blocks viral replication in animals (Nature, 2021, 595:596-599).
Proper condensation of epigenetic proteins is a crucial for chromatin regulation and cancer control
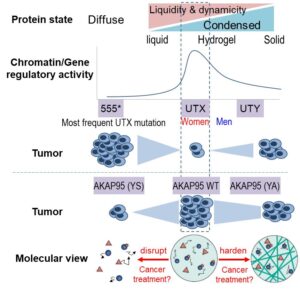 KDM6A/UTX is a H3K27 demethylase and an important tumor suppressor with elusive mechanism, as the demethylase activity is often dispensable in tumor suppression. We show that the tumor-suppressive activity of UTX is critically dependent on its IDR-mediated phase separation. By mutagenesis, IDR replacement, and genome editing, we show a critical role of UTX condensation in tumor suppression and ES cell differentiation. UTX recruits MLL4 and p300 into the co-condensates and enriches the H3K4 methylation activity of MLL4. UTX condensates regulate multi-level chromatin activity, including high-order chromatin interactions (looping), to orchestrate tumor-suppressive transcriptional programs. The Y chromosome homolog of UTX has weaker tumor suppressive activity as a result of forming more solid-like condensates driven by differential IDR sequence features. UTX condensation and condensate properties are often lost or altered in patients by mutations including single amino acid changes (Fig. 1). This work demonstrates a crucial function of liquid condensates in enabling the tumor-suppressive activity of a chromatin regulator, and that natural variations in condensates properties may contribute to cancer sex bias (Shi et al., Nature, 2021). These findings strongly suggest previously unappreciated sequence variations (especially the overlooked but prevalent disordered regions) in cancer-associated proteins in different population groups may alter protein condensation ability or condensate properties, thereby altering the cancer-regulating activity and contributing to cancer disparities.
KDM6A/UTX is a H3K27 demethylase and an important tumor suppressor with elusive mechanism, as the demethylase activity is often dispensable in tumor suppression. We show that the tumor-suppressive activity of UTX is critically dependent on its IDR-mediated phase separation. By mutagenesis, IDR replacement, and genome editing, we show a critical role of UTX condensation in tumor suppression and ES cell differentiation. UTX recruits MLL4 and p300 into the co-condensates and enriches the H3K4 methylation activity of MLL4. UTX condensates regulate multi-level chromatin activity, including high-order chromatin interactions (looping), to orchestrate tumor-suppressive transcriptional programs. The Y chromosome homolog of UTX has weaker tumor suppressive activity as a result of forming more solid-like condensates driven by differential IDR sequence features. UTX condensation and condensate properties are often lost or altered in patients by mutations including single amino acid changes (Fig. 1). This work demonstrates a crucial function of liquid condensates in enabling the tumor-suppressive activity of a chromatin regulator, and that natural variations in condensates properties may contribute to cancer sex bias (Shi et al., Nature, 2021). These findings strongly suggest previously unappreciated sequence variations (especially the overlooked but prevalent disordered regions) in cancer-associated proteins in different population groups may alter protein condensation ability or condensate properties, thereby altering the cancer-regulating activity and contributing to cancer disparities.
We will continue to work on connecting the fundamental mechanisms of phase separation to human disease that are based on dysregulation of chromatin and gene expression. The condensed states are considered fundamental states of protein widely sampled and adopted by our cellular proteome (Nature Cell Biology, 2021, 23:587-594), but we are only in the beginning of understanding these protein states.
Roles of H3K4 methylation in stem cell fate determination and cancer.
Genetic lesions in the Set1/Mll family of H3K4 methyltransferases are extensively associated with cancer. Our studies primarily using conditional knockout mouse models have shown the profound importance of Dpy30, a core subunit of these complexes, and its associated H3K4 methylation in the two-way transitions between the pluripotent and differentiated states, in maintaining the identity and differentiation of hematopoietic stem cells and postnatal neural stem cells, through transcriptional control of stem cell signature programs, energy metabolism, and genome integrity. Dpy30 partial inactivation does not affect mouse physiology or lifespan, but significantly impaired Myc-driven tumorigenesis. Myc thus hijacks this epigenetic pathway to promote cancer, creating “epigenetic vulnerability”, which we have exploited by developing Dpy30 inhibitors in the format of peptides and small-molecules (ongoing) for cancer treatment.
