Spindle assembly and Chromosome Segregation in Mitosis
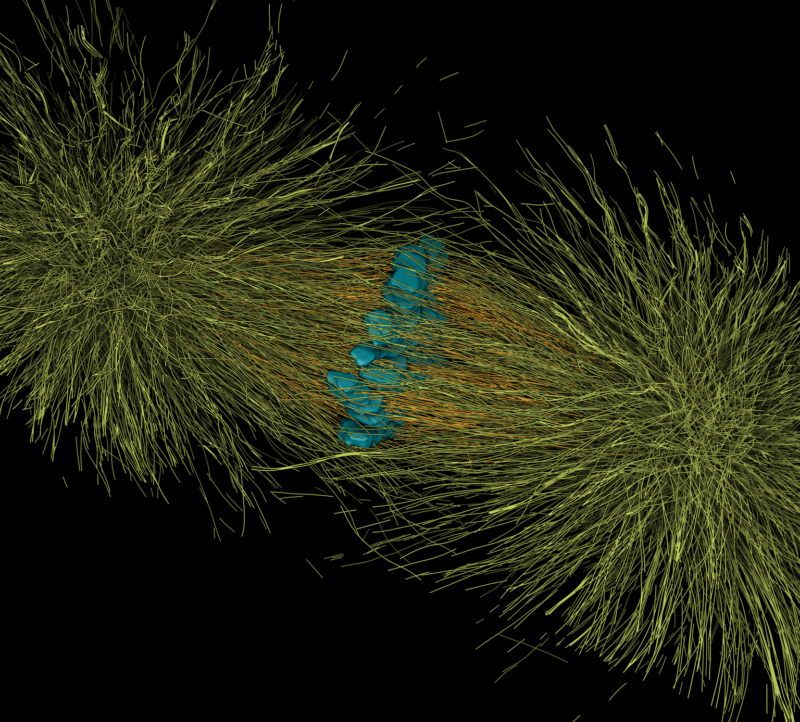 Mitotic spindles are self-organized structures constructed from dynamic microtubules, which faithfully segregate chromosomes to newly formed daughter cells. Errors in this process lead to wrong copy numbers of chromosomes (aneuploidy), which is detrimental for the organism. Aneuploidy is a hallmark for cancer.
Mitotic spindles are self-organized structures constructed from dynamic microtubules, which faithfully segregate chromosomes to newly formed daughter cells. Errors in this process lead to wrong copy numbers of chromosomes (aneuploidy), which is detrimental for the organism. Aneuploidy is a hallmark for cancer.
Chromosome separation is mainly driven kinetochore microtubules that are connected to specific regions on the chromosomes, the kinetochores. Previous research has provided compelling evidence that the integrity of microtubule attachments to kinetochores is essential for a faithful segregation of chromosomes. Defects in microtubule attachment to kinetochores result in aneuploidy.
We have currently two major projects in the lab focusing on chromosome segregation during mitosis.
The mechanisms of chromosome segregation during anaphase
 The segregation of chromosomes during anaphase is mediated by two mechanisms: Anaphase A, shortening of the pole to chromosome distance and Anaphase B, increase of the pole to pole distance. While anaphase A is well understood, less is known about anaphase B. Two major mechanisms have been proposed: Cortical pulling forces pulling on the spindle poles and/or sliding of antiparallel microtubules (MTs) in the central spindle. Although plus-end directed kinesins are thought to drive the sliding, the identity of the motor proteins involved in anaphase B has not been revealed. In addition, how the forces generated by MTs and motors are transmitted to the chromosomes is unknown. Currently, a major obstacle in the field are the lack of optical resolution allowing us to distinguish the interactions of MTs in the central spindle with each other, kinetochore microtubules (KMTs) and chromosomes, as well as the multiple functions of many of the key players of central spindle organization that impair the formation of proper metaphase spindles, preventing a reliable analysis of mechanisms of anaphase.
The segregation of chromosomes during anaphase is mediated by two mechanisms: Anaphase A, shortening of the pole to chromosome distance and Anaphase B, increase of the pole to pole distance. While anaphase A is well understood, less is known about anaphase B. Two major mechanisms have been proposed: Cortical pulling forces pulling on the spindle poles and/or sliding of antiparallel microtubules (MTs) in the central spindle. Although plus-end directed kinesins are thought to drive the sliding, the identity of the motor proteins involved in anaphase B has not been revealed. In addition, how the forces generated by MTs and motors are transmitted to the chromosomes is unknown. Currently, a major obstacle in the field are the lack of optical resolution allowing us to distinguish the interactions of MTs in the central spindle with each other, kinetochore microtubules (KMTs) and chromosomes, as well as the multiple functions of many of the key players of central spindle organization that impair the formation of proper metaphase spindles, preventing a reliable analysis of mechanisms of anaphase.
Using reconstructions of wildtype spindles during the first mitosis in C. elegans as reference data, we are studying the functional roles of selected proteins involved in chromosome segregation. We are investigating the direct effect of perturbations of de-/polymerases, motor-proteins, cross-linkers and other proteins on spindle morphology on a microstructural level using our unique approach of tomography, light microscopy and modelling.
Variations in Chromosomes and kinetochore size and microtubule binding capacity and the impact on faithful chromosome segregation
 Detailed analysis of cancers has shown that aneuploidies of distinct chromosomes are cancer-specific and that some chromosomes show reoccurring aneuploidies. In addition, there has been increasing evidence that chromosome missegregation is biased and some chromosomes missegregate much more frequently. The reasons for this bias have remained unsolved. We hypothesize that chromosome specific aneuploidy is at the basis of tumor initiation and that structural differences between chromosomes and their interaction with microtubules are major drivers of chromosome specific aneuploidy. Based on the observation that the individual chromosomes differ significantly in their size and structural arrangement of specific domains along the chromosome, it is reasonable to speculate that those structural differences contribute to changes in microtubule interactions leading to biases in chromosome segregation.
Detailed analysis of cancers has shown that aneuploidies of distinct chromosomes are cancer-specific and that some chromosomes show reoccurring aneuploidies. In addition, there has been increasing evidence that chromosome missegregation is biased and some chromosomes missegregate much more frequently. The reasons for this bias have remained unsolved. We hypothesize that chromosome specific aneuploidy is at the basis of tumor initiation and that structural differences between chromosomes and their interaction with microtubules are major drivers of chromosome specific aneuploidy. Based on the observation that the individual chromosomes differ significantly in their size and structural arrangement of specific domains along the chromosome, it is reasonable to speculate that those structural differences contribute to changes in microtubule interactions leading to biases in chromosome segregation.
While there is significant data on the molecular factors that facilitate aneuploidy, we know almost nothing about how the different physical and mechanical properties of chromosomes influence their segregation during cell division. As an example, we know that microtubules exert forces on chromosomes to initially position them and consequently divide them to the two daughter cells. While a long line of research has shown that failures in the correct attachment of microtubules to chromosomes lead to chromosome missegregation, we in fact do not know why only some chromosomes missegregate during mitosis but never all of them. What are the factors that increase the risk of missegregation in the first place and how do they affect individual chromosomes?
Our research utilizes cutting-edge technologies such as 3D tomography, light-microscopy and novel CRISPR approaches to quantify and dissect the impact of structural properties of chromosomes and microtubule attachments on chromosome segregation fidelity. This approach will provide unique and novel information and lay the groundwork for discovering the mechanisms of chromosome-specific aneuploidy and of faithful chromosome segregation.
More Reading:
Redemann, S., Fürthauer, S., Shelley, M., & Müller-Reichert, T. (2019). Current approaches for the analysis of spindle organization. Current opinion in structural biology.
Yu, C. H., Redemann, S., Wu, H. Y., Kiewisz, R., Yoo, T. Y., Conway, W., … & Needleman, D. (2019). Central-spindle microtubules are strongly coupled to chromosomes during both anaphase A and anaphase B. Molecular biology of the cell, 30(19), 2503-2514.
Baumgart, J., Kirchner, M., Redemann, S., Bond, A., Woodruff, J., Verbavatz, J. M., … & Brugués, J. (2019). Soluble tubulin is significantly enriched at mitotic centrosomes. Journal of Cell Biology, 218(12), 3977-3985.
Lindow N, Redemann S, Brünig F, Fabig G, Müller-Reichert T, Prohaska S. Quantification of three-dimensional spindle architecture.Methods Cell Biol. 2018;145:45-64. doi: 10.1016/bs.mcb.2018.03.012. PubMed PMID: 29957215.
Müller-Reichert T, Kiewisz R, Redemann S. Mitotic spindles revisited – new insights from 3D electron microscopy.J Cell Sci. 2018 Jan 30;131(3). pii: jcs211383. doi: 10.1242/jcs.211383. Review. PubMed PMID: 29382699.
Redemann S, Baumgart J, Lindow N, Shelley M, Nazockdast E, Kratz A, Prohaska S, Brugués J, Fürthauer S, Müller-Reichert T. C. elegans chromosomes connect to centrosomes by anchoring into the spindle network.Nat Commun. 2017 May 11;8:15288. doi: 10.1038/ncomms15288. PubMed PMID: 28492281; PubMed Central PMCID: PMC5437269.
