Change of Sponsor
Change of Sponsor
| Field | Instructions | Example | Tips/Notes | Screenshot |
|---|---|---|---|---|
| Short Title | Create a short description of the request that is identifiable in the Award Workspace. Do not use "MD-SURG-Smith" naming convention. | "Change of Sponsor from Company A to Company B" | Review existing AMRs in Award Workspace for consistency of title format | 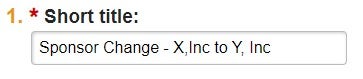 |
| Date Requested | Date of Submission/Request | 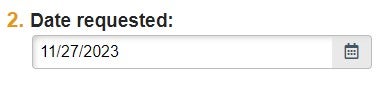 |
||
| Full Description | Include all information needed to complete the subsequent Mod request. | 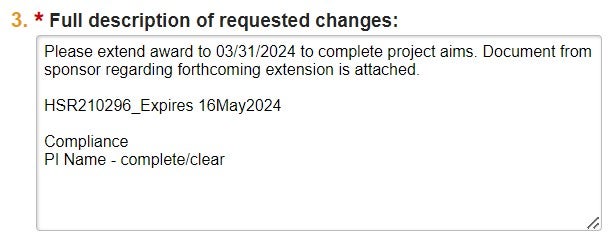 |
||
| 1. Describe full request details, including: 1a. Old and new sponsor names 1b. Effective date | "Please change sponsor from Company A to Company B, effective 03/01/2023, per the attached notification letter." | If the new sponsor is not in the Huron database, include the new sponsor address and contact info for OSP's review/vetting. | ||
| 2. Approval status (e.g. sponsor approval attached or letter/request attached for submission to sponsor) | "Please execute attached amendment to change the study sponsor from Company A to Company B." | |||
| 3. If necessary, contact info for Sponsor communication | This is required for all CTA or other bilateral agreements/amendments or if we are submitting a request to the Sponsor for the action. | |||
| 4. Compliance of all S/K Personnel | "Compliance: Person 1 - Complete/Clear Person 2 - Complete [SFI flag] Person 3 - Complete [EAD flag]" | Review personnel compliance via QlikSense "Research Conflicts of Interest" report. Please see instructions for accessing this report here. | ||
| Supporting Documents | 1. Sponsor Approval or Letter/Request | File naming convention: "001 - Confirmation of Sponsor Change" or "001 - Sponsor #_Current PI Name_Change of PI Request" | 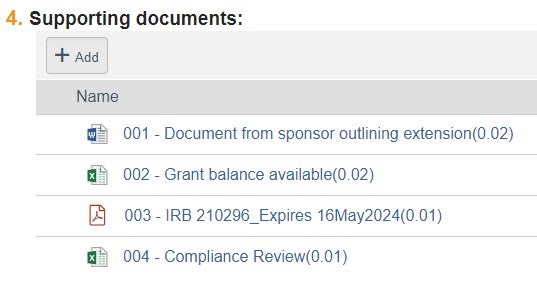 |
|
| 2. Compliance printout from QlikSense | File naming convention: "002 - Compliance Review" | |||
| Submitter | Assign Submitter as your SOMOGC administrator | DO NOT click "Submit to Specialist" | 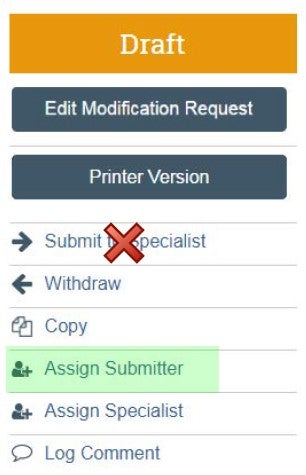 |
|
| Request Type [dropdown] | Change Sponsor | 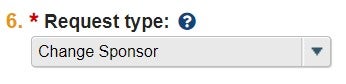 |
||
| General Tips/Notes | Send AMR # and/or link to somogc@uvahealth.org to get the action logged into the SOMOGC work queue. | Without this email, we will have no idea that your AMR is ready for review | ||
| PI and Department approvals should be captured via "Log Comment" activity. | If necessary, approvals can be provided outside of the system and uploaded as supporting documentation. See template here. | 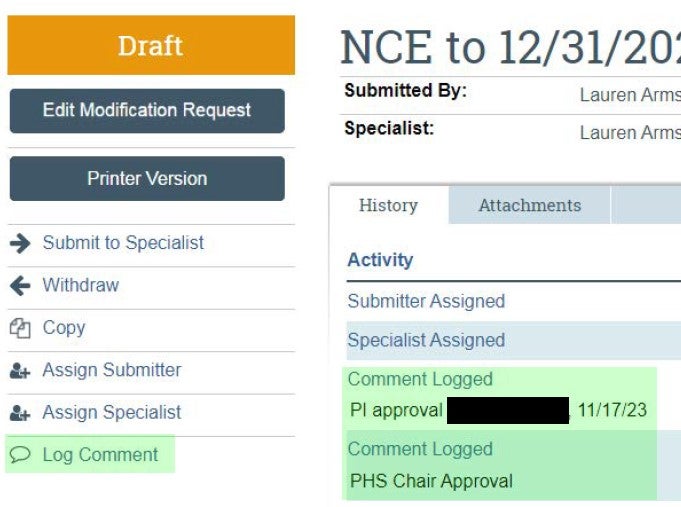 |
||
| For all AMR types, please include relevant email communication thread to provide context for the action being requested. | ||||
| Include all relevant attachments within SmartForm request page, not through "Add Comment" or on History tab. | ||||
| Don't use the WITHDRAW activity unless you really want to discard/trash the action. It cannot be reinstated once withdrawn. |
