Jiang Lab
Our Mission
At our lab, we have a strong emphasis on comprehending the pathophysiology of neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and other related dementia disorders. Our primary objective is to deepen our understanding of these disease conditions.
To achieve this, we employ cutting-edge technologies in disease modeling, allowing us to recreate and study the pathological processes in a controlled environment. By utilizing these advanced techniques, we gain insights into the intricate molecular mechanisms that underlie the progression of these diseases.
Moreover, our lab is dedicated to the development of innovative therapeutic strategies. We strive to translate our research findings into practical applications that could potentially lead to the discovery of novel treatments. By integrating our knowledge of disease mechanisms with targeted therapeutic approaches, we aim to make significant contributions to the field of neurodegenerative disease research.
Overall, our lab is committed to advancing the understanding, diagnosis, and treatment of neurodegenerative diseases through the utilization of state-of-the-art technologies and the exploration of molecular mechanisms.
Research
Our research focuses on several key areas that drive our scientific interests and contribute to the understanding and treatment of neurodegenerative diseases. These areas include:
- Investigation of RNA modifications and epitranscriptomic mechanisms in the pathogenesis of Alzheimer’s disease (AD) and related dementias. By studying how RNA modifications impact disease progression, we aim to uncover novel therapeutic targets and diagnostic markers.
- Exploration of neuron-glia interactions and neuroimmunological mechanisms underlying the progression of AD and Parkinson’s disease (PD). Understanding the complex interplay between neurons and glial cells will provide crucial insights into the underlying mechanisms and potential avenues for therapeutic intervention.
- Utilization of iPSC-induced 3D human brain organoid models to study neurodegenerative diseases and enable precision medicine approaches. These advanced models allow us to recapitulate the complexity of the human brain, facilitating the identification of disease mechanisms and the development of personalized treatment strategies.
- Elucidation of the molecular mechanisms driving prion-like propagation in the Braak stages of AD. Investigating the spread of pathological proteins in the brain will contribute to our understanding of disease progression and may lead to the development of interventions that disrupt this process.
- Development of innovative genetic and small molecule therapeutics for neurodegenerative diseases. Our research aims to identify and optimize potential therapeutic targets, including genetic approaches and small molecules, with the goal of ultimately translating these findings into effective treatments for patients.
These research directions represent our current focus and ongoing projects, which collectively contribute to the advancement of knowledge and the development of new strategies for the management and treatment of neurodegenerative diseases.
Projects
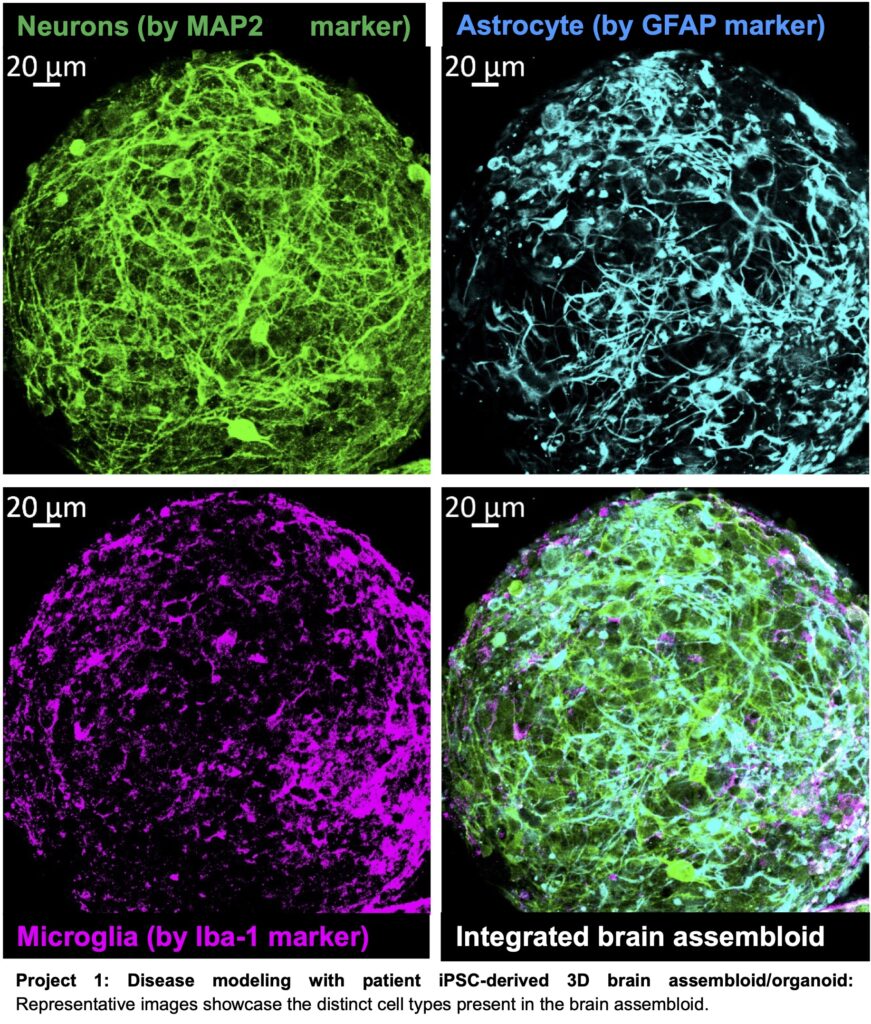
Our lab is actively engaged in a groundbreaking research project focused on the generation of 3D human brain assembloids/organoids. These models serve as invaluable tools for exploring neuron-glia interactions during both brain development and disease processes.
Alzheimer’s disease (AD) is a highly complex disorder with diverse contributing factors, such as age, gender, early-onset status, co-morbidities, family history, and ApoE genotypes, which vary significantly among different ethnic groups. This extensive heterogeneity has posed challenges in developing effective disease-modifying treatments for AD. To address this issue, we have conceived the idea of generating personalized brain assembloid models derived from AD patients with varied genetic backgrounds, including both familial and sporadic AD cases (Rickner HD and Jiang L et al., Nat Commun. 2022; PMID: 36271092).
Our primary goals are to elucidate the underlying disease mechanisms and investigate treatment regimens from the perspectives of precision medicine and regenerative approaches. Currently, we are in the process of establishing a 3D brain organoid model that incorporates four distinct cell types: neurons, astrocytes, microglia, and oligodendrocytes. This sophisticated model aims to recapitulate the pathogenesis of Alzheimer’s disease and enable us to explore the intricate molecular mechanisms driving disease progression.
By utilizing this brain assembloid model, we anticipate significant advancements in our understanding of neuron-glia interactions and neuroinflammation in the context of AD and related disorders. This research endeavor will provide valuable insights into the disease process and may pave the way for the development of targeted therapeutic strategies.
Overall, our research project on 3D human brain assembloids/organoids represents an innovative and promising approach to unraveling the complexities of AD and exploring novel avenues for treatment and intervention.
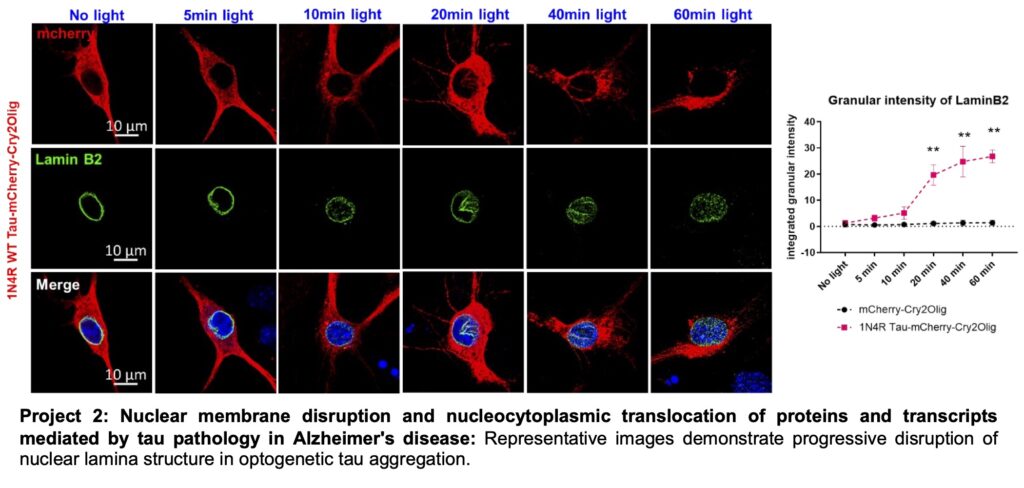
Misfolding and aggregation of microtubule-associated protein tau (tau) are core features of Alzheimer’s disease and related disorders (ADRD). The accumulation of aggregated tau closely correlates with cognitive decline. However, the mechanism of tau-induced neurotoxicity remains elusive. We have developed an optogenetic tool by fusing tau with the light-sensitive Cry2 structure to control and track protein aggregation in real-time (See video below). Additionally, we have combined optogenetics with proteomics to identify tau and protein interactomes with high temporal resolution. Our innovative studies have revealed that prolonged tau aggregation induces nuclear membrane disruption by binding to laminB and lamin B receptor, leading to the nucleocytoplasmic translocation of proteins and N6-Methyladenosine (m6A) modified RNAs (Jiang et al., Mol Cell, 2021; PMID: 34453888). Nuclear lamin plays a role in regulating cellular senescence and is directly or indirectly involved in DNA replication, transcription, reparation, and neuronal cell cycle re-entry in AD. Dysfunction of the nuclear lamina can disrupt nucleic acid and protein synthesis machinery. In the current project, our aim is to elucidate the transcriptomic and epitranscriptomic profiles sequestered by tau aggregation and identify the proteomic map of translocated proteins using TurboID proximity labeling. We will conduct our analysis using the P301S Tau transgenic mouse model of AD and 3D neuron-glial brain assembloid models derived from patient iPSCs. The findings will be validated using post-mortem human brain tissues, allowing for an integrative understanding of the pathogenesis of AD.
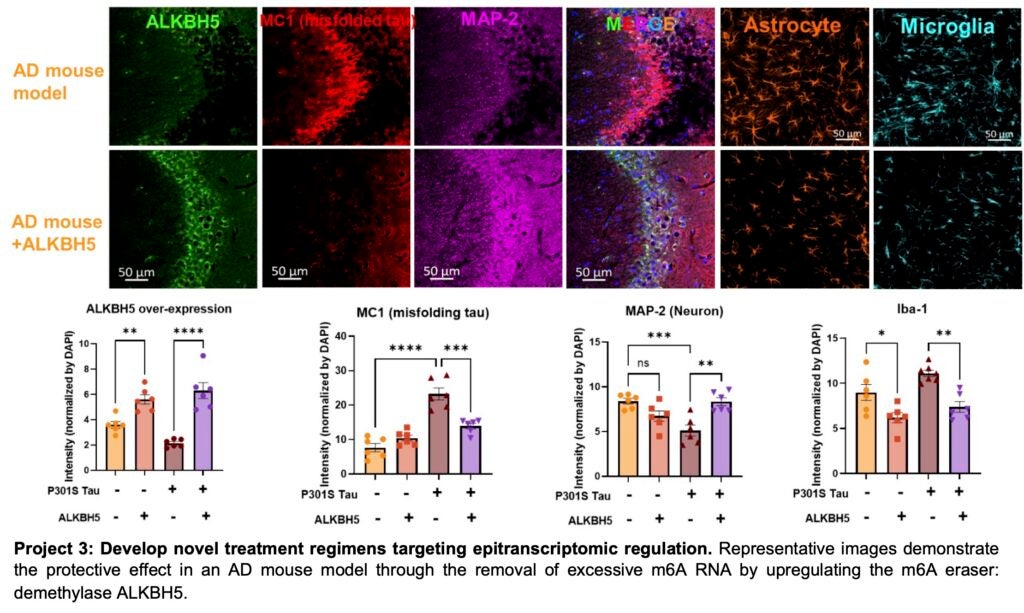
Recently, our research has uncovered an important connection between N6-Methyladenosine (m6A) modified RNA and the pathogenesis of Alzheimer’s disease (AD). We observed that m6A modified RNA is translocated from the nucleus to the cytoplasm, where it co-localizes with oligomeric tau (oTau) in post-mortem brain tissue of AD patients. As AD progresses to Braak stages 5 to 6, there is a sharp elevation in the m6A levels, leading to a 3-5 fold accumulation in the cytoplasm of affected neurons. This discovery is significant because m6A modification is the most common epitranscriptomic mark on mRNA and plays a crucial role in regulating protein synthesis and degradation. Furthermore, studies have demonstrated that RNA modifications have a profound impact on various cellular processes, including alternative splicing, microRNA metabolism, and protein recoding. These processes are particularly crucial for maintaining the proper functioning of important receptors in the central nervous system. Given these new findings, we are motivated to conduct a comprehensive investigation of epitranscriptomic changes in the progression of neurodegeneration.
Our primary objectives are twofold: first, to develop novel treatment regimens that target the dysregulated RNA metabolism in AD and other neurodegenerative diseases, and second, to explore the transcriptome in neurons and glial cells during the aging process using a human tau transgenic mouse model. In our ongoing research, we are specifically examining the involvement of m6A regulator proteins, including the demethylase ALKBH5 and the methyltransferase Mettl3, as potential targets for disease-modifying treatment strategies. By gaining a deeper understanding of the epitranscriptomic changes associated with neurodegenerative diseases, we hope to identify new therapeutic avenues for modulating RNA metabolism and ultimately develop effective treatments for AD and related conditions.