Translational Pipeline
Contact Us
Send EmailThe UVA Comprehensive Cancer Center Translational Pipeline is the mechanism by which ideas and discoveries generated by UVACCC members are developed and translated into products that can positively impact the lives of patients. The Translational Pipeline has two interconnected tracks: Clinical Trial and Commercialization.
To contact ORD regarding the Translational Pipeline, please click the button at right or email jck3xb@uvahealth.org.
Contact Us
Send EmailThe “Full Circle” of Translational Research
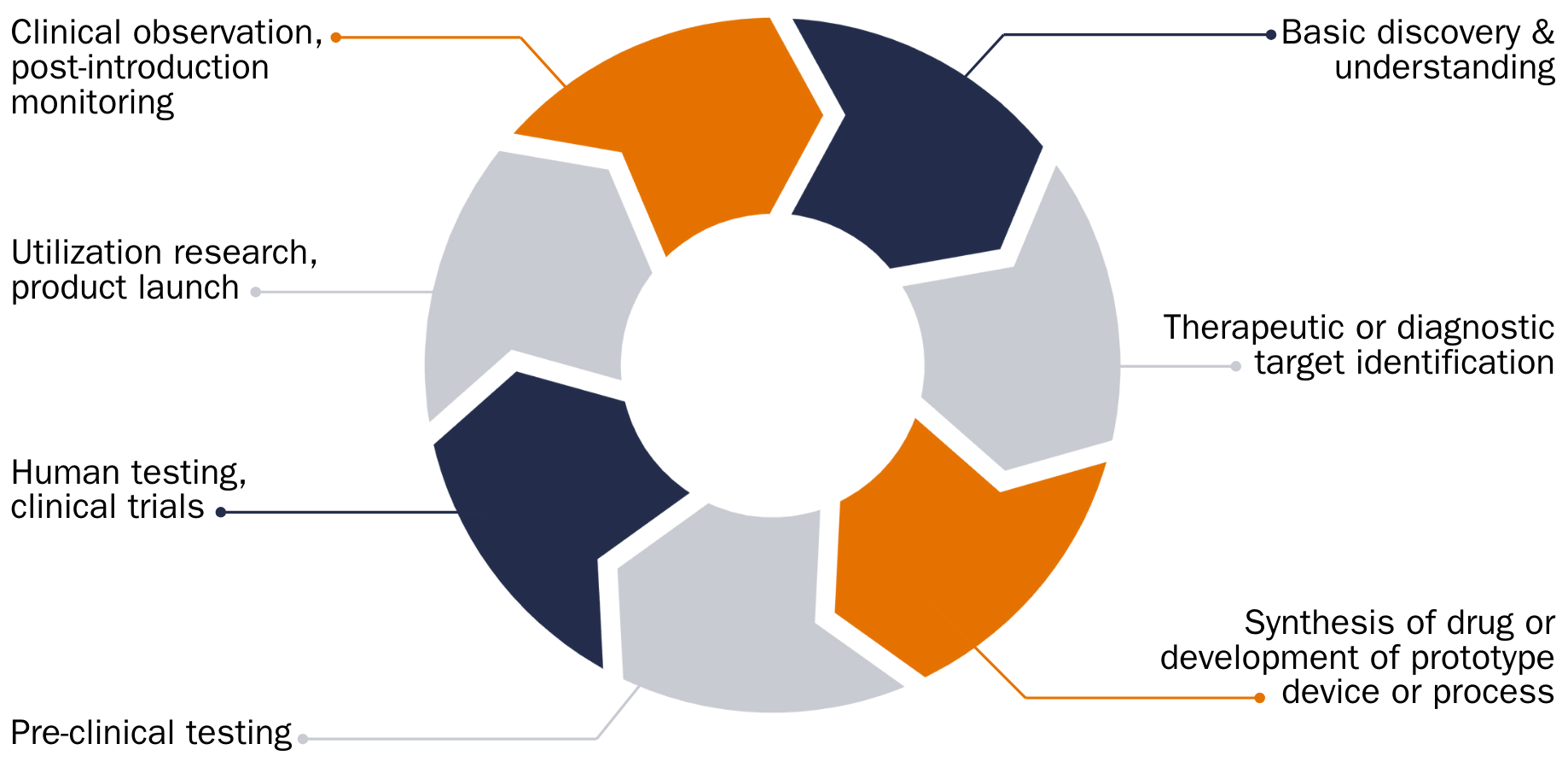
Clinical Trial Track
Projects on the Clinical Trial Track can emerge from the Commercialization Track or from ideas and data that support repurposing and/or combining approved treatments for new indications. Members interested in developing an investigator-initiated clinical trial (IIT) are encouraged to get feedback from the Cancer Therapeutics Program (CRX) and appropriate Translational Research Teams (TRTs), if they exist. The Cancer Center has a robust clinical trials infrastructure to enable all aspects of a clinical trial beginning with writing the protocol and including all required aspects to the end of the study. Information about clinical trial resources can be found through the following links:
Commercialization Track
Projects in the Commercialization Track are those associated with intellectual property and enter the Translational Pipeline either at the time of disclosure or when an investigator indicates an interest in pursuing patent protection for their project. The Cancer Center created the Accelerating Innovation Fund to support innovative, entrepreneurial cancer-relevant biomedical research projects with commercialization potential. For more information, click here. Additional resources to support commercialization activities can be found through the following links:
