Resources
Partner in Discovery offers researchers the opportunity to make use of patient biospecimens, molecular data, and clinical data. In addition, we also offer clinical research services.
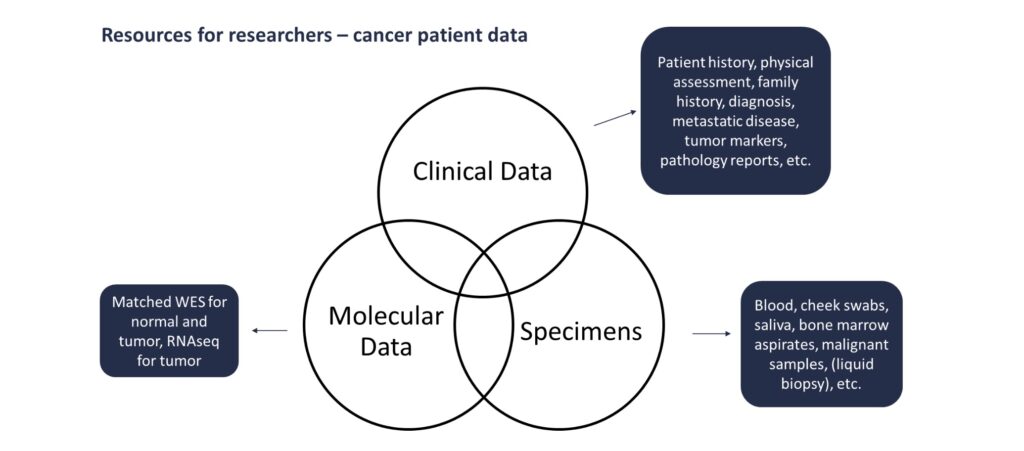
Includes information such as patient history, physical assessment, family history, diagnosis, metastatic disease, imaging, tumor markers, labs, radiation, etc.
Whole exome sequencing of 19,396 genes at 300x coverage, including ~440 cancer genes sequenced at 600x average depth for both paired germline and tumor specimens. RNAseq for tumor specimens. Standard of care genomics can be abstracted on a case-by-case basis.
Blood, buccal swabs, frozen tissue, FFPE, bone marrow aspirates, left over surgical tissues, etc.
Genomics Data Resource
Large datasets like TCGA, GTEx, CCLE are now available for access on our local Rivanna Project Drive. The only ask is that you acknowledge the Cancer Center in future publications as well as inform us of these publications.
For access, please contact PID Program Director, Elizabeth Mulcahy at qx3k@uvahealth.org.
| Folder Name | Data availability | Access time (and requirements) |
|---|---|---|
| UVACC_public | CCLE RNAseq, WES, WGS, and mass spec | Same day |
| UVACC_TCGA | All available RNAseq | Days (current dbGAP approval) |
| UVACC_GTEx | All available RNAseq | Days (current dbGAP approval) |
| ORIEN_Avatar | 325 clinical data elements, WES_n, WES_t, and RNAseq_t for ~900 UVA cancer patients | Days (project request,* signed data usage agreement, IRB exempt form** if de-identified) |
*To access the Partners in Discovery Project Request Form, click here.
**To access the IRB exempt form, click here.
Clinical Research Services
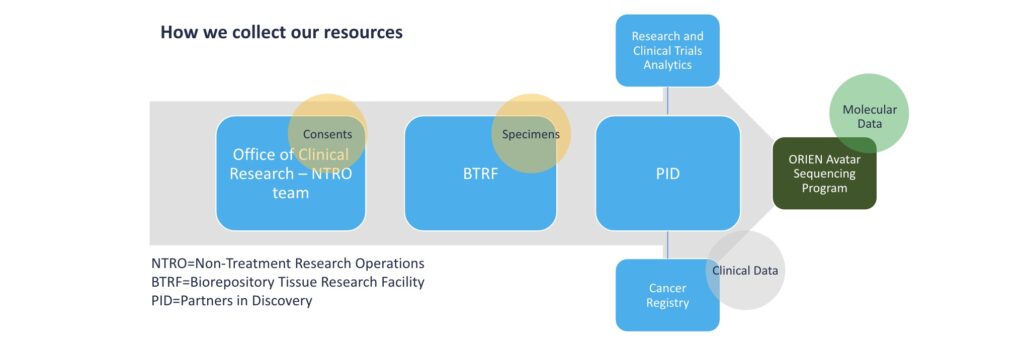
Through the biobanking efforts of the Partners in Discovery team and UVA’s Biorepository and Tissue Research Facility (BTRF), our investigators will have access to these services:
- Pre-screening of potential study participants
- Compliant consent of study participants
- Survey administration and phone follow-up
- Specimen coordination, processing and shipping
- Chart review/case report form completion
- Please go here to complete a project request with the NTRO team.
- Data abstractions from EPIC and Cancer Registry to complete case report forms
- Coordinate upload of EPIC data extractions into REDCap or other data capture systems
- Queries on internal and external Partners in Discovery databases for project feasibility
- Coordinate with Research and Clinical Trial Analytics Team and Cancer Registry for project data needs
- Feasibility for grant applications
- Feasibility for clinical trials for actual number of qualifying patients
- Access to whole exome sequencing and RNA sequencing for tumor and whole exome sequencing for germline samples
- Project-specific access to raw molecular files for consented patients
- Query processed data using Partners in Discovery informatics modules and ORIEN CBioPortal
- Project submissions for access to molecular data from other ORIEN sites
Studies may either rely on the specimen management that is part of the Partners in Discovery protocol through the Partners in Discovery core functions or may rely on an independent protocol with consent with unique specimen criteria. For those studies that rely on the Partners in Discovery protocol, the following table provides the collection details.
Specimens |
Tube Type |
Collection Volume |
Collection Limit |
Products |
Comments |
| Blood from solid tumor patient | K2EDTA | 10 mL | Cumulative blood collection total of 60 mL/8wk | 1 mL whole blood stored for future germline sequencing, remainder stored as buffy coat and 1 mL plasma aliquots | Modification to usual collection or processing generate additional charges |
| Blood from blood cancer patient | Na heparin | 20 mL | Cumulative blood collection total of 60 mL/8wk | Viably frozen mononuclear cell aliquots | Modification to usual collection or processing generate additional charges |
| Blood from blood cancer patient: For Circulating tumor DNA | Streck tube | 10 mL | Cumulative blood collection total of 60 mL/8wk | Determined on project specific basis | Project specific basis and fees apply |
| Bone marrow aspirates | Sodium heparin | 10 mL | 10 mL/4wk | Viably frozen mononuclear cell aliquots | |
| Buccal swabs | MAWI DNA kit | 1 swab kit | 2 per year | Cell suspension | |
| Lymph Node FNA
Extra pass (blood cancers only) |
2 extra passes | Snap frozen for cores, RPMI for FNA |
