Protocol Review Committee Members & Procedures
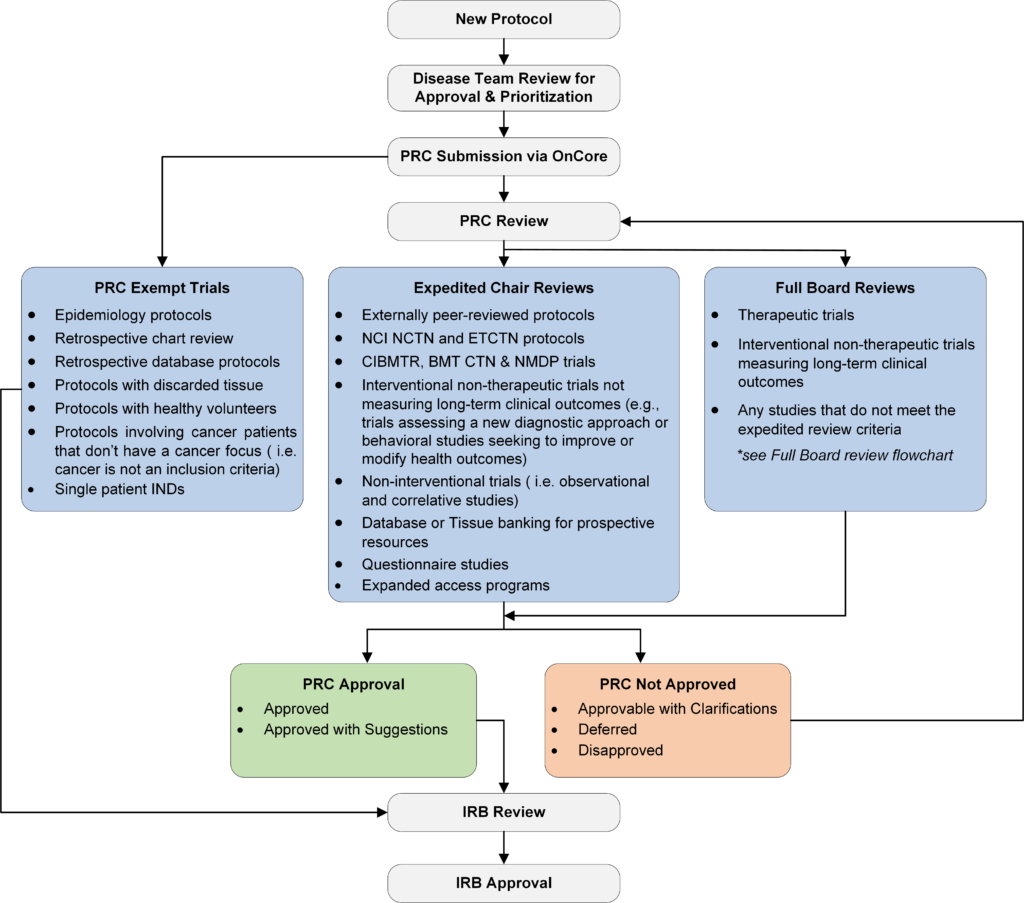
All pharmaceutical, cooperative group, and investigator-initiated protocols involving cancer patients or cancer-relevant studies must receive a review by the PRC. Examples include: studies about cancer risk factors, prevention, cancer treatment, survivorship or studies that include participants currently or previously diagnosed with cancer or their caregivers.
- The PRC meets on the second Thursday and fourth Monday of every month. The deadline for submission is 12:00 pm. two weeks prior to the meeting
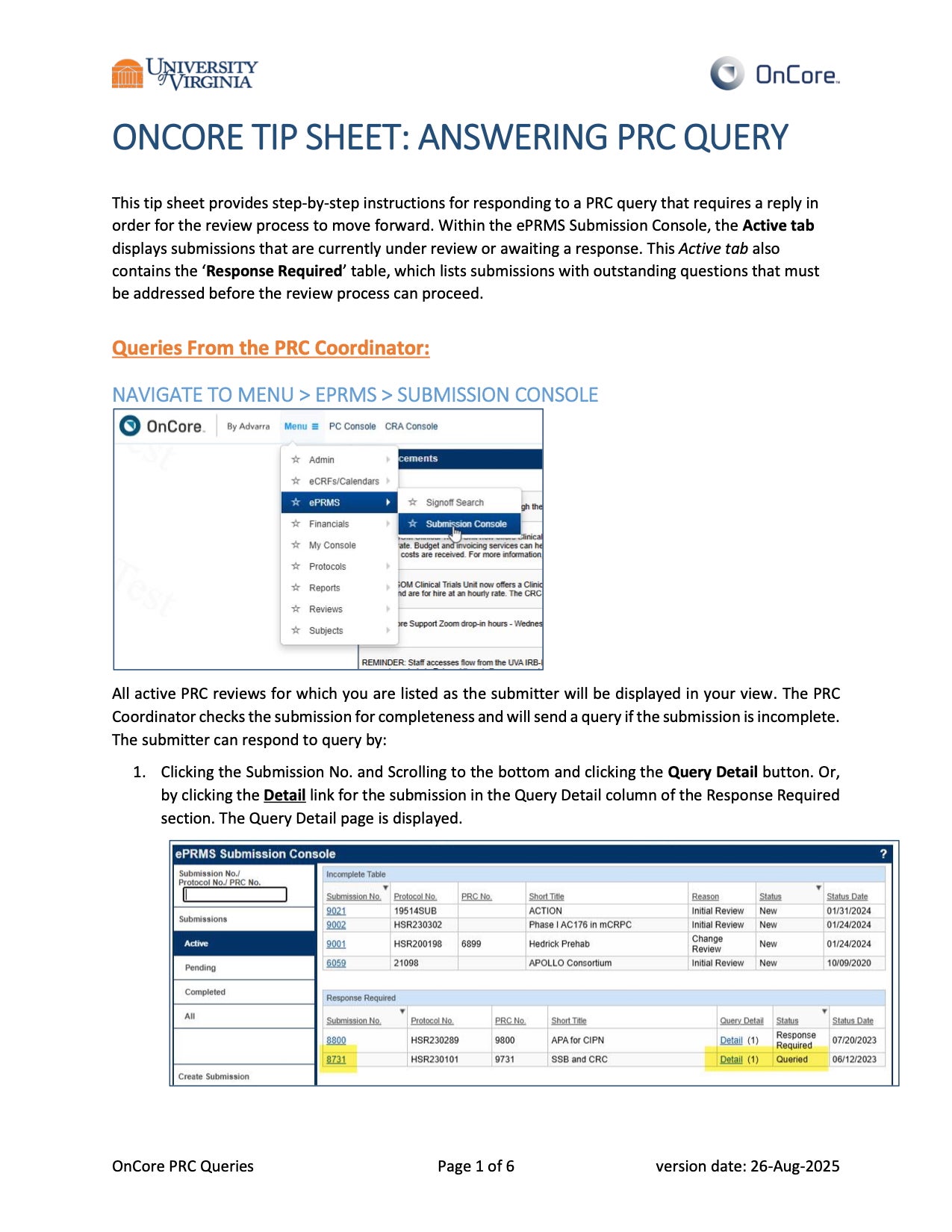
- PRC submissions should be completed via the OnCore ePRMS Submission Console. Please reach out to OnCore Support (ONCORESUPPORT@uvahealth.org) for system access or help with protocol submission to the PRC. We also recommend viewing the PRC Submission Digital Training Module.
- Cancer Prevention & Population Health (CPH) investigators may complete the PRC Submission form in REDCap. Once the form is completed, the PRC Coordinator will reach out with next steps.
- For each new interventional (therapeutic or non-therapeutic) or non-interventional protocol submission to the PRC, the PI/Study team is required to submit the following documents:
- Study Protocol
- PRC Submission Form
- Investigator Drug Brochure/ Investigator Device Brochure (if applicable)
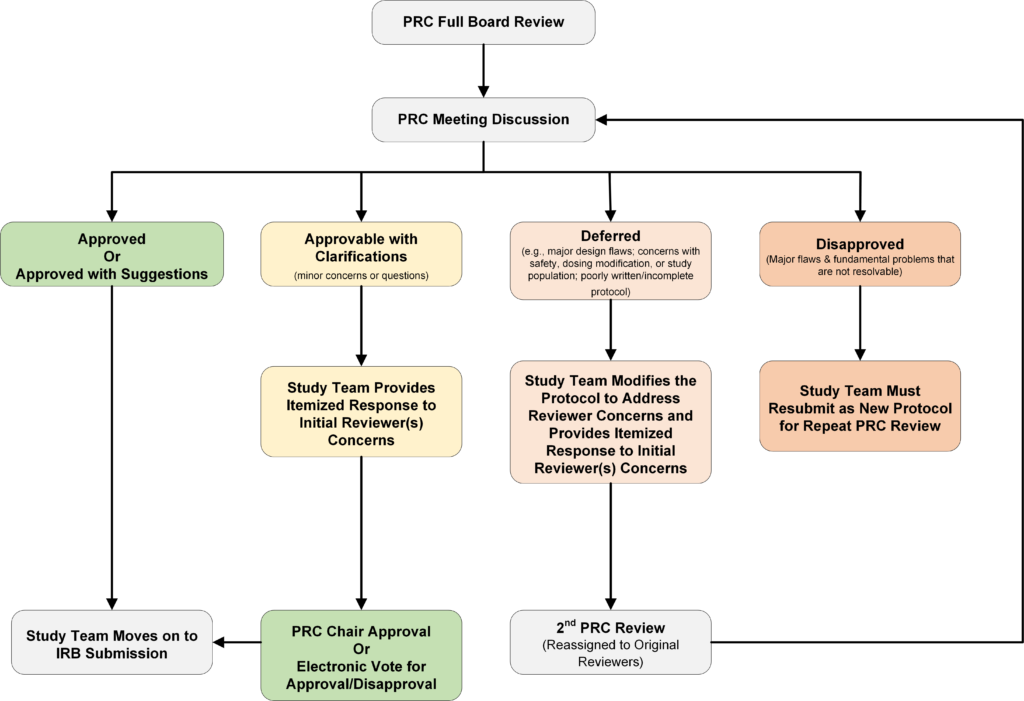
- The PRC Chair with the assistance of the PRC coordinator determines if protocol requires full board review, or if it’s eligible for administrative/expedited review, or if it’s exempt from PRC review.
- The PRC assigns risk classification level for all new protocols. This also dictates the level of CC DSMC monitoring for UVA IITs.
- After the meeting, PRC decision letters are uploaded in the OnCore ePRMS Console and an auto-generated notification email with the letter is sent to the investigator.
- When revisions and/or responses are required, the study investigator is expected to respond within 30 days. The protocol will be considered under PRC review process until it is approved, withdrawn by the study team, or if more than 6 months have elapsed between the initial PRC review and the study team’s response.
- Submission to the Institutional Review Board for Health Sciences Research will require a final approval letter from the PRC Chair/Co-chair.
- Once a protocol has undergone an initial PRC review and approval, all subsequent protocol revisions/modifications require PRC review and approval (except NCI Cooperative group and exempt protocols).
- If you are unsure your protocol amendment is exempt from PRC review, please email the PRC Coordinator your protocol amendment with the summary of changes. We will review the document(s) and provide a response.
- PRC amendment submissions should be completed via the OnCore ePRMS Submission Console. Please reach out to OnCore Support (ONCORESUPPORT@uvahealth.org) for system access or help with amendment submission to the PRC. We also recommend viewing the PRC Submission Digital Training Module.
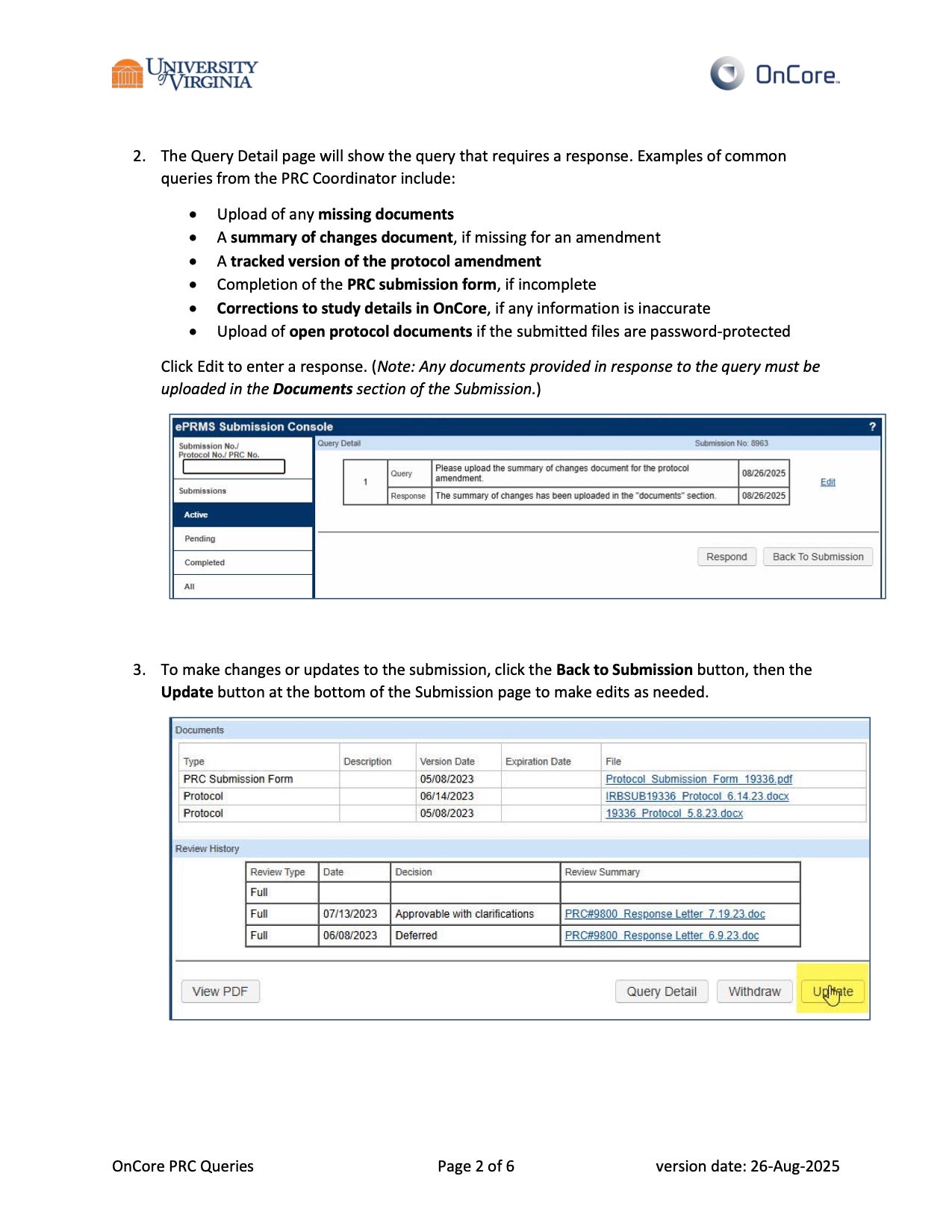
- When submitting revisions to a protocol, please ensure that a tracked change version and a summary of changes are included in the submission.
- Post PRC approval, the following types of protocol amendments and document updates are exempt from PRC review:
- Personnel updates/changes
- Simple clarifications to protocol language or administrative changes
- AE reporting clarifications
- Important safety concerns (proceed to IRB)
- Modifications/amendments for cooperative group and other externally peer-reviewed protocols that have already received PRC approval
- Recruitment materials updates/changes
- Consent form updates/changes
- Investigator’s brochure updates/changes
- The PRC monitors accrual to all cancer-related clinical trials that enroll human subjects or that use clinical specimens that can be linked to individual patient or participant data. Accruals are reviewed at PRC semi-annual performance review meetings in May and November. PRC may close non-accruing trials and a letter with this recommendation is sent to the PI.
- Please note: PRC will provide a letter of exemption for any new protocols that do not require PRC review. Email the PRC coordinator if you believe your study should be exempt from PRC review.

If you have questions, please contact Saba Mahmood at sm4pp@uvahealth.org.
PRC Members
Ryan Gentzler, MD
Hematology Oncology
PRC Chair, Department of Medicine
Emily Ayers, MD
Hematology Oncology
PRC Co-chair, Department of Medicine
Roger Abounader, MD
Microbiology, Immunology & Cancer Biology and Neurology
Ludimila Cavalcante, MD
Hematology Oncology
Michael Devitt, MD
Hematology Oncology
Patrick Dillon, MD
Hematology Oncology
Michael Keng, MD
Hematology Oncology
Paul Vincent Viscuse, MD
Hematology Oncology
Christopher McLaughlin, MD
Radiation Oncology
Kari Ring, MD
Gynecologic Oncology
Kara Romano, MD
Radiation Oncology
Krithika Shanmugasundaram, MD
Hematology Oncology
David Shonka, MD
Otolaryngology
Shayna Showalter, MD
Surgical Oncology
Phil Chow, PhD
Center for Behavioral Health & Technology
Soutik Ghosal, PhD
Department of Public Health Sciences, Division of Biostatistics
Debamita Kundu, PhD
Department of Public Health Sciences, Division of Biostatistics
Hong Zhu, PhD
Department of Public Health Sciences, Division of Biostatistics
Michal Ande, CCRC
Clinical Research Coordinator 3
Ashley Donihee
Clinical Research Coordinator 2
Cara Hanby, BS, CCRP
Protocol Development Specialist
Alexandra Cash, BS, CCRP
Clinical Research Coordinator 2
Yaroslav Shvorak
Clinical Research Coordinator 2
Andrew Whitman
Pharmacist
Alecia Guishard
Pharmacist
Morgan Phillips
Pharmacist
Michael Laporte
Pharmacist
Maxwell Herbert
Pharmacist
Lisa Trinh
Pharmacist
Saba Mahmood
Senior Compliance Analyst
