Acoustic Neuromas (Vestibular Schwannoma)
About
An acoustic neuroma (also known as vestibular schwannoma) is a benign, typically slow growing tumor that develops from the balance portion of cranial nerve VIII. The tumor comes from an overproduction of Schwann cells–the cells that normally wrap around nerve fibers serve as insulation to the nerves. As the vestibular schwannoma grows, it presses against the hearing and balance nerves, usually causing unilateral (one-sided) or asymmetric hearing loss, tinnitus (ringing in the ear), dizziness, and imbalance. As the tumor grows, it can interfere with the face sensation nerve (the trigeminal nerve), causing facial numbness. Rarely, vestibular schwannomas can compress the facial nerve (for the muscles of the face) causing facial weakness or paralysis on the side of the tumor. If the tumor becomes large, it will eventually press against nearby brain structures (such as the brainstem and the cerebellum) and cause more severe neurological deficits.
What is the difference between unilateral and bilateral vestibular schwannomas?
Unilateral vestibular schwannomas affect just one ear. They account for approximately 8 percent of all tumors inside the skull. One out of every 100,000 individuals per year develops a vestibular schwannoma. Symptoms may develop at any age but usually occur between the ages of 30 and 60 years. Unilateral vestibular schwannomas are not hereditary.
Bilateral vestibular schwannomas affect both ears and are hereditary. Bilateral vestibular schwannomas occur in individuals with a genetic disorder known as neurofibromatosis–type 2 (NF2). Affected individuals have a 50 percent chance of passing this disorder on to their children. Unlike those with a unilateral vestibular schwannoma, individuals with NF2 usually develop symptoms in their teens or early adulthood. In addition, patients with NF2 usually develop other brain and spinal cord tumors. For instance, they also can develop tumors of the nerves important for swallowing, speech, eye and facial movement, and facial sensation. Determining the best management of the vestibular schwannomas as well as the additional nerve, brain, and spinal cord tumors is more complicated than deciding how to treat a unilateral vestibular schwannoma.
Diagnosis
Early diagnosis of a vestibular schwannoma is important to prevent serious consequences. In more than 70% of the patients with vestibular schwannomas, hearing loss and tinnitus are the first symptoms. In most patients there is a gradual decrease in hearing with difficulty in understanding spoken words often encountered. Dizziness, vertigo and headache are less common symptoms. The ringing or “tinnitus” may be constant or change with activity. In patients with very large tumors additional symptoms may include weakness of facial muscles, double vision, hoarseness, facial pain or numbness or difficulty swallowing.
 Hearing loss is shown in over 90% of patients the first time medicalattention is sought. The audiogram is important to determine the speech reception threshold (SRT), speech discrimination (SD) and pure tone average (PTA). The pattern of hearing loss may suggest nerve injury and include high frequency hearing loss in excess of low frequency loss.
Hearing loss is shown in over 90% of patients the first time medicalattention is sought. The audiogram is important to determine the speech reception threshold (SRT), speech discrimination (SD) and pure tone average (PTA). The pattern of hearing loss may suggest nerve injury and include high frequency hearing loss in excess of low frequency loss.
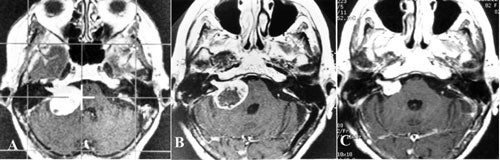
The appearance of the vestibular schwannoma on the MRI is a rounded, enhancing mass with extra-canalicular (outside the internal auditory canal), intra-canalicular, or both components. A cyst may be present within the acoustic neuroma.
The diagnosis is confirmed with a high degree of accuracy by the characteristic appearance on MRI. As misdiagnosis is rare, confirmation by biopsy is not generally needed.
Treatment Options
- Surgical resection
- Stereotactic radiosurgery
- Monitoring without treatment.
Surgery
The exact type of operation done depends on the size of the tumor and the level of hearing in the affected ear. If the tumor is very small, hearing may be saved. As the tumor grows larger, surgical removal is more complicated because the tumor may have thinned-out the nerves that control facial movement, hearing, and balance and may also have affected other nerves and structures of the brain.Surgery offers immediacy but with higher up-front risk. The risks of surgery include cerebrospinal fluid leak (5% to 17% risk), meningitis, hydrocephalus, and a wound infection. Other common effects include a period of headaches or balance difficulties. Other potential risks of surgery include facial weakness. This risks increase with size of the tumor and the type of surgical procedure utilized.
Three surgical approaches are commonly utilized. When there is a desire to attempt to preserve useful hearing, the retrosigmoid or middle cranial fossa approaches are often utilized. With these approaches hearing may be preserved for a substantial proportion of patients (especially with smaller tumors) but hearing loss remains a significant risk. When there is no useful hearing or hearing is to be sacrificed, the translabyrinthine approach is often considered. Although hearing is lost with the latter operation, the risk of facial nerve injury may be reduced. The relative merits of each of these approaches depends upon the individual anatomy, size and location of tumor, presence of useful hearing, and individual patient concerns/expectations.
Radiosurgery
Radiosurgery offers effective treatment. The goal of radiosurgery is tumor volume control (i.e. the inhibition of tumor growth) and not tumor removal. The risks of radiosurgery are also less than for surgery. For radiosurgery, the frame is applied to the patient’s head, a scan is taken and the radiation is given in one large “shot.” With radiosurgery the tumor control rate is approximately 95%.
Observation
As acoustic neuroma may be slow growing, observation with delayed treatment may be acceptable for some patients. This is especially the case for elderly or infirm patients with mild symptoms where the risks of therapy may be greater and where the tumor may not grow during their lifespan. Those with smaller tumors may also decide upon watchful waiting, getting frequent MRI’s and audiograms. In this situation it is highly likely that treatment would eventually be needed, but there is potential to postpone therapy for many years. Treatment would proceed when growth is confirmed or symptoms worsen.
For an individual considering this approach, the risks of tumor growth causing symptoms or making later treatment more difficult should be compared with the potential benefits of delaying treatment along with its risks and side effects. Lifelong MRI and audiogram follow-up are needed so that treatment might be given if the tumor grows.
Choosing among the options
Microsurgery and radiosurgery are appropriate choices for the majority of patients, but the decision must be quite individualized. We recommend consultation both with specialists in neurosurgery prior to deciding upon a treatment course. For those interested in a consultation, we will review records and scans submitted to us and provide individualized information about which approach may be best.
History of Gamma Knife treatment
The first vestibular schwannomas treated with the gamma knife were by Leksell and Steiner in 1969. Since then more than 21,272 have been treated around the world through June 2003. The indications for gamma knife surgery for this tumor vary. Some physicians advocate gamma knife surgery in medically high risk patients, patients who refuse microsurgery, and in patients with post-operative residual tumor. However, others advocate gamma knife surgery as the treatment of choice in nearly all cases of vestibular schwannomas.
The usefulness of irradiation in the post-operative period was shown by Wallner in 1987 where external beam irradiation lowered the recurrence rate from 46 to 6 percent in Boldrey’s surgical series at the University of California at San Francisco. By then, gamma surgery was already being widely applied to this disease under many circumstances. The fact is that for a number of reasons few neurosurgeons acquire the necessary competency to satisfactorily extirpate these tumors. This situation may change if a method to improve the acquisition of skills required for extirpation of these lesions is found.
The advent of MRI has made planning for this procedure much more exact. With a high quality MRI scan and a relatively small tumor, the seventh cranial nerve can occasionally be visualized and carefully excluded from the treatment field. The trigeminal nerve can nearly always be identified except with the largest tumors, which in most cases should not be treated primarily with gamma surgery.
Small collimators are used to better match the isodose configuration to the size and shape of the tumor. We have had no brainstem related complications. Previously we used minimum periphery doses up to 20Gy and maximum doses up to 70Gy. Presently, we use a margin dose of 11 to 15Gy at the 30 to 50 percent isodose curve. The incidence of cranial nerve palsies rose considerably at the higher doses without significant improvement in the degree of tumor control.
Studies at UVA Gamma Knife
At the University of Virginia, we have treated 400 patients with vestibular schwannomas. One-hundred fifty-three of these patients with greater than twelve months follow-up have been reported. Of these radiosurgery was the primary treatment for 96 and was adjutant (following microsurgery) in 57. The volume of the treated tumors ranged from 0.02 to 18.3 cm3.
Of the patients treated primarily with Gamma surgery, a decrease in tumor size was seen in 81 percent (78 patients), no change in 12 percent, and an increase in size in 6 percent. Among those 78 patients with a decrease in the size of their tumors, the decrease was greater than 50 percent in 20 patients. It is our policy to not consider decreases in volume of less than 15 percent as significant. This is true of all tumors and vascular malformations that we treat. Radiological follow-up for these patients ranged from 1 to 10 years.
Of the 57 patients treated with Gamma surgery after microsurgery, a decrease in tumor size was seen in 65 percent, no change in 25 percent, and an increase in size in 10 percent. Among the 37 patients with a decrease in the size of their tumors the decrease was greater than 50 percent in 12 patients. The outcome in terms of post-radiosurgical volume reduction in patients who had prior microsurgery is not as favorable as those who were primarily treated with Gamma surgery. This difference is likely a result of the increased difficulty with accurate targeting in those who have undergone prior microsurgery. Of note, although our experience with treating large vestibular schwannomas is small (n=19), we have observed a 95% tumor control rate in these following gamma surgery.
In our patients, there were five with transient changes in trigeminal sensation and three with facial paresis. One of the patients with facial weakness was operated upon shortly after Gamma surgery and was lost to follow-up. Another patient recovered completely in six weeks, and the third has nearly completely recovered at ten months. Of the patients with useful hearing prior to gamma surgery, 58% retained their hearing following radiosurgery, 42% experienced some degree of deterioration, and 31% lost useful hearing. The majority of hearing changes were observed at the 2-year checkup, and additional auditory changes were observed as late as 8 years post-radiosurgery.
Other centers report similar rates of tumor control (i.e. with no change or decrease in the size of the tumor) seen in 89 to 100 percent of patients.
Evaluation of the material from the Karolinska group included evaluation of radiographic changes besides size. The most common change was loss of central enhancement within the tumor on either contrasted MRI or CT studies. This occurred in 70 percent of patients and typically was observed within 6 to 12 months of treatment. However, these changes were reversible. Another change that was observed and that we have often seen is a transient increase in the size of the tumor during the first 6 months after Gamma surgery. This is commonly seen in tumors that then regress to their original size or smaller.
Previously published incidence of cranial neuropathies at other centers was 17 percent at Karolinska and 29 percent at Pittsburgh for facial paresis which in the vast majority of cases was transient or mild. The tri
geminal nerve was affected in a variety of ways in 33 percent of the time at Pittsburgh, most commonly a mild hypoesthesia. Recent complication rates at these institutions are comparable to those at our center.
We have not seen an instance of cerebellar edema or hydrocephalus requiring spinal fluid diversion following Gamma surgery for vestibular schwannomas, but both of these have been reported elsewhere.
Outcome of Radio Surgery for Acoustic Neuromas
| Series | No. of Patients w/follow-up Imaging | Avg Follow-up(mos.) | Tumor Increase(%) | Tumor Unchanged or Decreased (%) |
|---|---|---|---|---|
| Noren et al. (1993) | 209 | minimum of 12 | 16 | 84 |
| Flickinger et al. (1993) | 134 | 24 | 11 | 89 |
| Foote et al., (1995) | 35 | 16 | 0 | 100 |
| Kwon et al. (1998) | 63 | 52 | 5 | 95 |
| Steiner et al. (2000) | 153 | 51 | 7 | 93 |
| Flickinger et al. (2001) | 190 | 30 | 3 | 97 |
| Bertalanffy et al. (2001) | 40 | 36 | 9 | 91 |
| Iwai et al. (2003) | 51 | 60 | 4 | 96 |
