Arteriovenous Malformations (AVM)
Arteriovenous malformations (AVM) are congenital disorders in which connections between veins and arteries are tangled, making them prone to bleeding that can be fatal.
Click here for our page on large AVMs.
Click here for information about Causes, Symptoms, Diagnosis and more treatment options.
Technical Information from UVA studies
 The minimal clinical and only moderate changes in the normal cerebral vasculature after high doses of gamma radiation is in sharp contrast to the response of the vessels of an AVM. Complete radiographic obliteration can be achieved after appropriate Gamma surgery. The effects of ionizing radiation and a role in the management of AVM’s was first reported in 1928 by Cushing. During craniotomy for an AVM he had to interrupt surgery due to major hemorrhage from the lesion. He then treated the patient with fractionated radiation. At re-operation five years later only an obliterated avascular mass was discovered. This early success was overshadowed by numerous series of failures. In this early period, Johnson was the only one to report reasonable results with a 45% angiographic obliteration. The introduction of the Gamma Knife rekindled interest in the treatment of AVM’s with radiation.
The minimal clinical and only moderate changes in the normal cerebral vasculature after high doses of gamma radiation is in sharp contrast to the response of the vessels of an AVM. Complete radiographic obliteration can be achieved after appropriate Gamma surgery. The effects of ionizing radiation and a role in the management of AVM’s was first reported in 1928 by Cushing. During craniotomy for an AVM he had to interrupt surgery due to major hemorrhage from the lesion. He then treated the patient with fractionated radiation. At re-operation five years later only an obliterated avascular mass was discovered. This early success was overshadowed by numerous series of failures. In this early period, Johnson was the only one to report reasonable results with a 45% angiographic obliteration. The introduction of the Gamma Knife rekindled interest in the treatment of AVM’s with radiation.
The pathological changes in AVM’s treated with the Gamma Knife have been described by several authors. The earliest change is damage to the endothelium with swelling of the endothelial cells and subsequent denution or separation of the endothelium from the underlying vessel wall. The most important changes are seen later in the intima with the appearance of loosely organized spindle cells (myofibroblasts) and an extracellular matrix containing collagen type IV, not seen in the intima of untreated vessels. Expansion of the extracellular matrix and cellular degeneration define the final stage prior to luminal obliteration. The occlusion of the vessels is not a thrombotic process but rather the culmination of concentric narrowing of the vessel by an expanding vessel wall. Subsequent recanalization of an angiographically proven obliterated AVM has not occurred in our experience.
The indications for gamma surgery of AVM’s versus other treatment options is in many cases unclear at best. Small asymptomatic inoperable AVM’s are clearly best treated with the Gamma Knife while AVM’s with a large symptomatic hemorrhage in non-eloquent superficial brain are best treated with open surgery. The reason for this is that the risk-benefit value is clear in both of these situations. In other situations it is more ambiguous. A knowledge of the capabilities of various treatments to effect cure, the associated morbidity and mortality associated with the treatment and the natural history of the disease following various treatments must be known to accurately prescribe the most efficacious treatment plan. Unfortunately these are in most instances not known. The natural history of AVM’s is not fully understood. Some authors believe that size matters, with smaller AVM’s bleeding at a higher rate than larger ones or at a lower rate. There is also evidence that size is independent of the hemorrhage rate. Similarly the rate of hemorrhage of an AVM following a previous hemorrhage is thought to be higher than the rate in unruptured AVM’s by some authors but not by others. The effects of age, gender, pregnancy and AVM location also confound the question of risk of rupture.
The results of microsurgery published in the literature tend to come from centers of excellence and the patients they treat with open surgery are, by definition more amenable to this treatment. The effectiveness of the treatment by this manner and its co-morbid results are known shortly after surgery. The short term morbidity of treatment with the Gamma Knife approaches zero but because the benefit and potential complications require time to become apparent follow-up of these patients is problematic. The quality of the AVM’s treated with the Gamma Knife also varies in large series with those treated by microsurgery. All of these make comparison of the modalities difficult. Add to that the additional risks and benefits of preoperative embolization and the matter is that much less clear. It is paramount to the physician treating a patient harboring an AVM to be aware, as much as possible, of the options that are available and the magnitude of the risks and benefits associated with each.
Results
We have treated 2,155 vascular malformations of which 1,917 were AVM’s with the Gamma Knife since 1970. As experience with this tool grows the capabilities and limitations of the Gamma Knife are being defined. Serendipitously the first AVM’s  were treated by prescribing a 25 Gy periphery dose. That means the edge of the AVM received this dose and that this was the minimum dose received by the entire AVM. Subsequent changes in protocol showed a significant decrease in success with doses less than 23 Gy and small improvements in obliteration rates but with significantly more radiation associated complications with higher doses. Optimally, therefore we treat most AVM’s with 23 to 25 Gy at the margin. With doses higher than 25 Gy, we did not observe any added benefit.
were treated by prescribing a 25 Gy periphery dose. That means the edge of the AVM received this dose and that this was the minimum dose received by the entire AVM. Subsequent changes in protocol showed a significant decrease in success with doses less than 23 Gy and small improvements in obliteration rates but with significantly more radiation associated complications with higher doses. Optimally, therefore we treat most AVM’s with 23 to 25 Gy at the margin. With doses higher than 25 Gy, we did not observe any added benefit.
As in microsurgery, also when performing Gamma surgery, feeding arteries or draining veins should be left alone and only the nidus should be treated. In very large AVM’s, only partially treated due to the excessive dose necessary to treat optimally, occasional cures have been achieved. This is thought to be due to fortuitous inclusion of all the pathological shunts within the higher dose treatment field. Targeting only the feeding vessels to the AVM has had very limited success because of recruitment of small angiographically occult feeding arteries. Interestingly, the first patient ever treated had only the feeding vessels targeted and a cure was obtained. The early success with this strategy has not been reproduced.
The results of Gamma surgery on AVM’s is affected by the minimum dose applied to the AVM and the size of the AVM. These two factors are interdependent. It has been shown that the limitation of the allowed margin dose by the size of the malformation decreases the rate of obliteration. There are reports contending that low dose Gamma surgery with large malformations results in obliteration rates that are comparable to smaller lesions treated with a larger margin dose. It is doubtful that these results will hold up with larger series. Thus far at our center, larger AVM’s have had a lower obliteration rate.
Between 1970 and 1990, 880 patients were optimally treated. Optimally is defined as at least 25 Gy at the margin of the entire nidus of the AVM. Of these patients the age range was 3 to 76 years. Approximately 15% were pediatric patients (<17 years old).
The presenting symptoms were hemorrhage (70 percent), seizures (16 percent), headache (5 percent), neurological deficits not associated with acute hemorrhage (4 percent), and other symptoms (2 percent)
The majority of referrals were for AVM’s deemed operable only with unacceptable morbidity explaining the fact that 73 Percent of the AVM’s were located in deep areas of the brain (20 percent in the basal ganglia or thalamus, 16 percent around or within the ventricular system, 11 percent within the brainstem) or within eloquent cortex.. Eighty five percent were supratentorial and among these 62 percent were located on the right side, 37 percent on the left side and 1 percent were centrally located.
In this series the patients treated earlier were subjected to a vigorous protocol of repeated angiograms. Later with the introduction of computerized tomography and then magnetic resonance imaging, angiography was not performed until nidus was no longer evident on these screening examinations.
Imaging Outcome
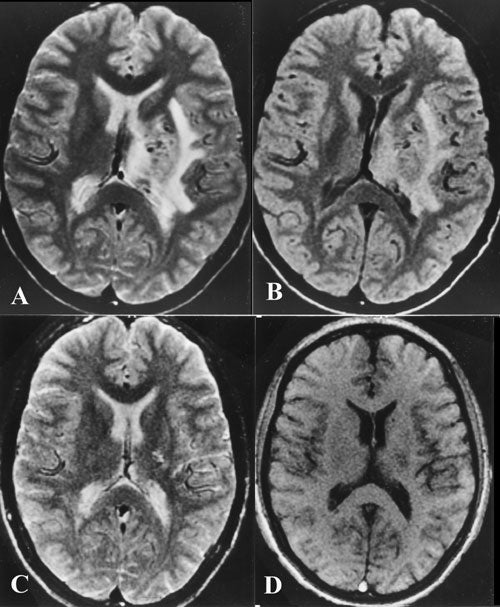
Of the 880 patients treated 461 had adequate angiographic follow-up. Of these 461 patients 80% were found to be cured within two years. In patients with MRI examinations that showed no residual nidus roughly 1/3 were found on subsequent 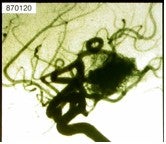 follow-up angiography to be cured, 1/3 to be subtotally obliterated (no residual nidus but early filling vein present on angiography) and 1/3 to be partially obliterated (nidus smaller but still present). Rarely, delayed formation of a cyst on MRI occurs at the site of an obliterated AVM; this infrequent occurrence has been reported by others.
follow-up angiography to be cured, 1/3 to be subtotally obliterated (no residual nidus but early filling vein present on angiography) and 1/3 to be partially obliterated (nidus smaller but still present). Rarely, delayed formation of a cyst on MRI occurs at the site of an obliterated AVM; this infrequent occurrence has been reported by others.
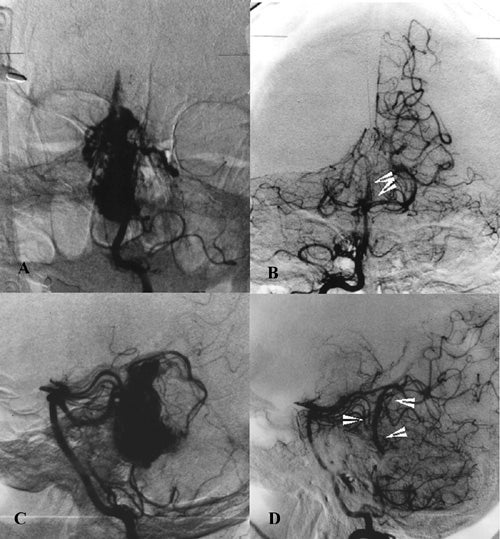
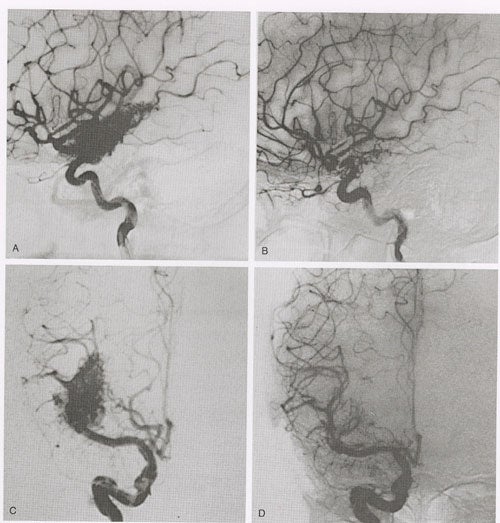
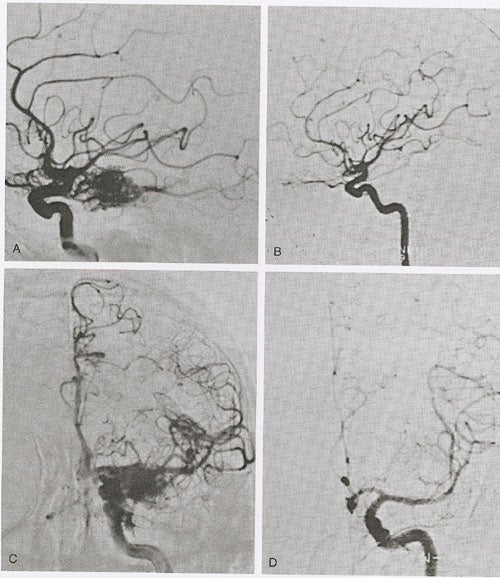
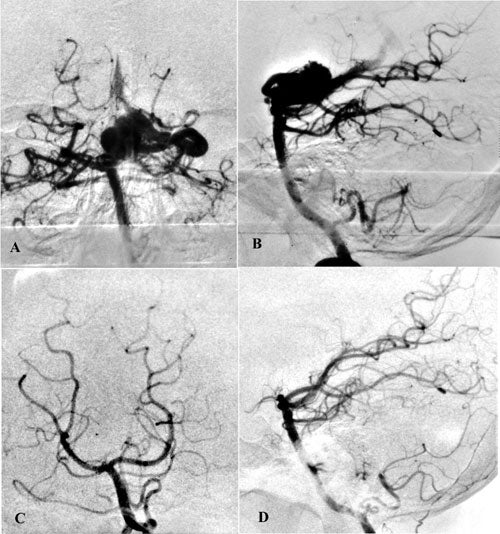
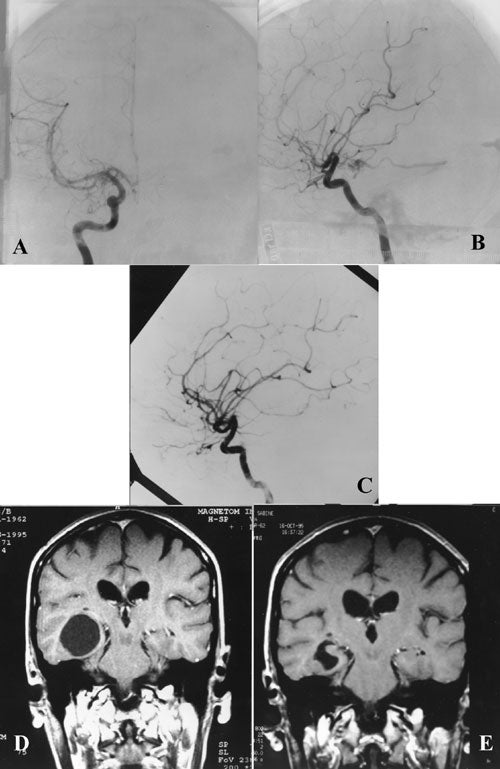
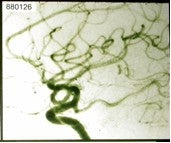
Angiographic changes precede obliteration. The diameter of the nidus as well as the feeding arteries and occasionally draining veins become smaller. The nidus decreases in size, and the shunt is reduced. We have classified angiographic changes as either no change, partial obliteration (decrease in the size of the nidus)(fig 2 ), subtotal obliteration (no evidence of nidus but with a persistent early draining vein) (fig 3 ) and total obliteration.(fig 4,5,6 )
At the time of the last evaluation of our results only 5 percent of patients who had had follow-up angiograms had no change in the status of their AVM’s. Eighty percent were cured, 10 percent had subtotal obliteration and 5 percent were partially obliterated. The angiogram should be complete, of high quality, and should be reviewed by an experienced and interested neuroradiologist or neurosurgeon. No patient that was harboring an angiographically proven obliterated AVM has ever hemorrhaged in our experience. Nor has a patient with a subtotally obliterated AVM sustained a post-radiosurgery hemorrhage. Regardless of this we do not consider a patient cured until he has total obliteration of the AVM. The early draining vein represents persistence of the shunt.
In this group of patients obliteration rates were affected by the size of the nidus. The rate for AVM’s less than 1 cubic centimeter in volume was 88 percent. For 1 to 3 cm3 it was 78 percent and for greater than 3 cm3 it was 50 percent.
Evaluation of patients treated sub-optimally (periphery dose less than 23 to25Gy) shows a sharp decline in obliteration rates.(fig 7 ) Doses greater than 25GY were associated with little improvement in obliteration rate.
Of the 2500 patients we have treated, 277 had embolization of the AVM prior to Gamma surgery. Only 53 had follow up angiograms. Of these 43 (81 percent) were cured. The low rate of follow-up angiograms in this subset is due disproportionately to evidence of flow voids on follow-up MRI scans. We are now undertaking a study of this subgroup including patients treated up to two years ago. It is clear however that the ability of the embolization procedure to improve obliteration rates with Gamma surgery depends upon shrinking the size of the nidus. If the embolization only decreases flow or splits the AVM into multiple portions it is unlikely to alter the outcome of Gamma surgery.
Gamma surgery was performed on 218 patients to treat residual nidus following microsurgery. Of these patients 182 had an angiogram at two years after Gamma surgery and 153 (85 percent were cured). Volume appears to be directly related to higher long-term complication rates.
Recently, there has been much discussion of prospective, staged treatment of large AVM’s (volume >15 cc) with Gamma surgery. Staging is typically designed to separate out 50% of the volume. Our results for large AVM’s indicate an obliteration rate of approximately 16%. At the University of Vienna, Kitz and his team have reported angiographically proven obliteration in 13 out of 14 patients with large AVM’s. If there is residual AVM, retreatment is possible. However, complication rates for retreatment are higher than initial treatment; rates range from 14% reported by Karlsson to 2% in our series. The wide variation in retreatment complication rates likely depends upon differences in the initial and residual AVM treatment volumes in each center’s experience.
There has been criticism leveled against radiosurgery series reported in the literature for AVM’s that the results are biased because angiography is only performed after there is no evidence of nidus residual on MRI. And because the reported obliteration rate is of the patients who had follow-up angiograms, the reported numbers are biased towards a favorable group. To a limited extent this is true. Evaluation of the group of 880 patients showed that assuming the worst case scenario where every patient with an MRI but not an angiogram was considered not cured the final percentages of obliteration dropped less than 1 percent.
Clinical Outcome
A review of the long term clinical outcome following Gamma surgery was carried out on 247 patients we treated between 1970 and 1983. The presenting symptoms widely varied and 94 percent of the patients had hemorrhaged prior to therapy. Ninety-eight of these patients had chronic headaches and 66 percent had complete relief following Gamma surgery. An additional 9 percent improved. Twenty six percent had seizures prior to therapy and 19 percent of these became seizure free and 51 percent improved. Eleven patients (5 percent of patients without a prior seizures had at least one seizure following therapy). Resolution or significant improvements were also seen in 53 of 74 patients with motor deficits (72 percent), 19/46 with a sensory deficit (41 percent), 23/44 with memory disturbance (52 percent) and 26/35 with language dysfunction (74 percent).
We have recently analyzed in detail the outcome of seizures in our patients by questionnaires. Of 1200 respondents, there were 178 patients with seizures as a preoperative symptom. At the time of analysis 62 percent were seizure free with almost half of these off medication. An additional 24 percent were improved.
The cause for clinical improvement following Gamma surgery in such a large number of patients is unknown. The natural history of neurological deficits to improve over time must be presumed to play a major role. The improvement in regional blood flow following AVM obliteration may also be responsible for a portion of the gains made by the patients. Whatever the reason, significant improvement is seen in many patients.
Hemorrhage Risk in the Treatment-Response Interval
The annual incidence of hemorrhage in untreated AVM’s is reported by various authors as between two and five percent. From research based on our own material we recently reached several other conclusions. The risk of hemorrhage increases with age and is also higher in females during the fertile years.
Whether Gamma surgery without obliteration of the nidus provides partial protection from hemorrhage is still controversial. It has been demonstrated by some authors that there may be some degree of protective effect. Because the incidence of hemorrhage in a matched group of untreated patients will likely never be known and the timing of obliteration is not known except as being between diagnostic scans, it is a difficult position to support.
The incidence of hemorrhage following Gamma surgery during the first two years was studied in 1604 of our patients and  reported by Karlsson. There were 49 hemorrhages for an annual incidence of 1.4 percent. This is significantly lower than the generally accepted rate of 3 to 4 percent per year but includes all 1604 patients, including those known and not known to have obliterated AVM’s. Of these hemorrhages 14 were fatal (annual rate of 0.4 percent) and 9 had permanent neurological deficits (annual rate of 0.3 percent).
reported by Karlsson. There were 49 hemorrhages for an annual incidence of 1.4 percent. This is significantly lower than the generally accepted rate of 3 to 4 percent per year but includes all 1604 patients, including those known and not known to have obliterated AVM’s. Of these hemorrhages 14 were fatal (annual rate of 0.4 percent) and 9 had permanent neurological deficits (annual rate of 0.3 percent).
In 113 patients with subtotal obliteration of the AVM we observed no hemorrhages. No hemorrhage occurred during 948 risk years (average 8.5 years). Assuming a natural risk of hemorrhage of 4% there should have been 34 hemorrhages in this interval. These observations should be tested on larger patient material before final conclusions are made. Moreover, others have noted AVM reappearance after apparent Gamma Knife surgery obliteration. In our histopathological analysis, Gamma surgery of AVMs caused endothelial damage, proliferation of smooth-muscle cells, and the elaboration of extracellular collagen by these cells, which led to progressive stenosis and obliteration of the AVM nidus. In this same report, there was evidence of small trapped vessels which would have very little blood flow. It is unclear what histopatholgical process would permit the formation of new vessels following radiosurgical-induced obliteration of the AVM nidus. However, it is clear that the infrequent and small trapped vessels observed by Schneider et al. (1997) could not explain the angiographic findings reported by Linqvist et al. (2000). Such inconsistent findings following radiosurgical treatment of AVM’s suggest the need for further clinical and histopathological investigation and the continued follow-up of patients, particularly pediatric ones, following Gamma surgery.
Complications
Acute post treatment nausea and vomiting occurs occasionally and is not associated with any lasting effects. These may be treated symptomatically.
Rare seizures have occurred in our experience, all in the post treatment period and in patients with a previous seizure disorder. None of our patients experienced a seizure while the head was secured within the gamma unit. However this possibility should not be discounted because of the risk of cervical spine injury associated with the head being fixed within the unit.
The results of a series of 816 patients treated for AVM’s with gamma surgery with follow up CT (630 patients) and/or MRI (239 patients) showed white matter changes in a significant number of cases. CT showed hypo-intensity changes in 11 percent of cases and increased signal on the T2 weighted images on MRI in 37 percent. The higher percentage in MRI scans represents the higher sensitivity of this imaging modality. The earliest onset of these changes was 3 months, and 92 percent occurred within 15 months. Resolution of these changes was the usual course, on average within 5 months (range 1 to 17 months).(fig 8 )
Clinical symptoms were associated with these changes in 6 percent of the entire group and resolved completely in one half. Therefore 3 percent of patients suffered a fixed neurological deficit following Gamma surgery for AVM.
A rare occurrence following gamma surgery for AVM’s is the development of an expansive cyst at or adjacent to an obliterated AVM. First reported in 1992 in two patients out of a series of forty, we have seen this event twice in our material. These occurred 5 to 10 years following treatment and occasionally are symptomatic due to mass effect requiring extirpation. The etiology of these cysts is unclear. There have been seven cases that we know of worldwide, five of which were operated upon, usually because of mass effect. There was no evidence of malignancy or tumor noted in any of these cases. All those reported were in patients imaged solely with angiograms at the time of treatment and the condition of the surrounding brain at the time of Gamma surgery was not documented by CT or MRI.(fig 9) We have preliminary data concerning the occurrence of this adverse effect in 1203 consecutive patients from a study in progress including 2500 cases of AVM’s treated between 1970 and 2002 with Gamma surgery. The incidence of cyst formation in the entire 1203 patients was 1.6% and 3.6% in patients with more than 5 years follow-up. Radiation induced tissue change following Gamma surgery and embolization were statistically related to the cyst formation.
Outcome of Radio Surgery for Arteriovenous Malformations
| Series | No. of Cases | Angiographic Obliteration % | Radiation- Induced Complications | Rebleeding |
|---|---|---|---|---|
| Bunge et al† | 374 | 82 | 4 | 5 |
| Sutcliffe et al(1992)[272] | 160 | 76 | 3.8 | 3.8 |
| Kawamoto et al‡ | 144 | 70 | 3.7 | 4.2 |
| Kondziolka et al (1993)[273] | 402 | 71 | 3.1 | 5.2 |
| Steiner et al (1979) | 461 | 80 | 3.1 | 2.3 |
| Chang et al. (2000)[274] | 277 | 79 | ||
| Karlsson, Lindquist, and Steiner (1997) [275] | 1319 | 85 | ||
| Flickinger et al.(1996)[276] | 197 | 72 |
*Angiographically proven obliteration.
†H. Bunge, personal communication, 1993.
‡S. Kawamoto, personal communication, 1994.