Exclusive Human Milk Diet Reduces Surgical NEC, BPD, and Severe ROP
Natalie Goldfield, Patti Perks RD, Jonathan Swanson MD MSc
Abstract
Background:
Extremely premature infants are at high risk for the development of several severe morbidities including bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), and necrotizing enterocolitis (NEC). There is ample evidence that suggests that nutrition, human milk (HM) in particular, plays an integral part in preventing these outcomes. To meet the nutritional needs of very low birth weight (VLBW; BW <1500 g) infants, HM is traditionally supplemented with human milk fortifier (HMF). Growing evidence suggests that VLBW infants fed with cow’s milk (CM) derived products have a greater risk of adverse outcomes. In 2016, the neonatal intensive care unit (NICU) at UVA Children’s Hospital instituted human-milk derived HMF for infants born 1250 g or less with the goal of reducing NEC.
Methods:
Infants were included in our analysis who were born between 2014 – 2020 with birthweight ≤ 1250 grams. We excluded those admitted > 48 hours from birth, died within 48 hours of birth, or who were found to have congenital gastrointestinal disease. All infants were fed a base diet of HM (mom’s milk or pasteurized donor milk). Infants were fortified with either a liquid CM-HMF (2014-2016) or HM-HMF (2016-2020). There were no significant changes occurred in feeding practices from 2014-2020 except for fortifying at 80 ml/kg/d instead of 120 ml/kg/d when starting HM-HMF. Infants were transitioned to either a preterm formula or CM-HMF at 34 weeks’ gestation.
Results:
Table 1 demonstrates our demographic characteristics of the two cohorts. Those fed a diet fortified with CM-HMF tended to be younger as well as had a higher rate of small for gestational age status (BW < 10th percentile).
Table 1: Demographic characteristics by cohort. Mean +/- SD or N (%)
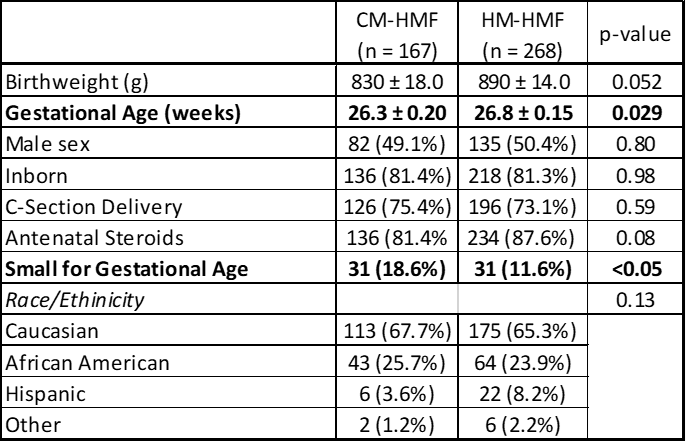
Table 2 provides survival and infectious outcomes by cohort. Infants fed a diet fortified with HM-HMF had a significant reduction in surgical NEC as well as peritonitis (culture-proved peritoneal culture). There were no statistical differences in other infectious outcomes.
Table 2: Infectious outcomes by cohort. Mean +/- SD or N (%)
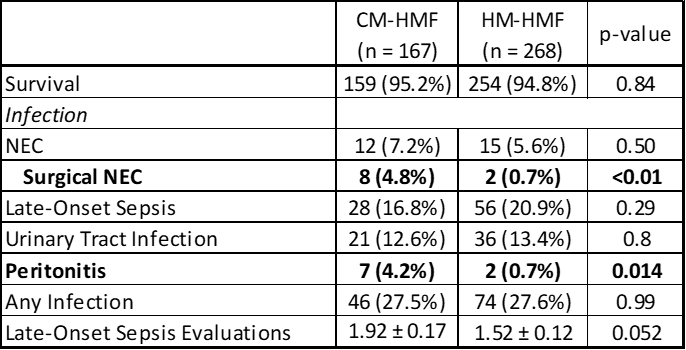
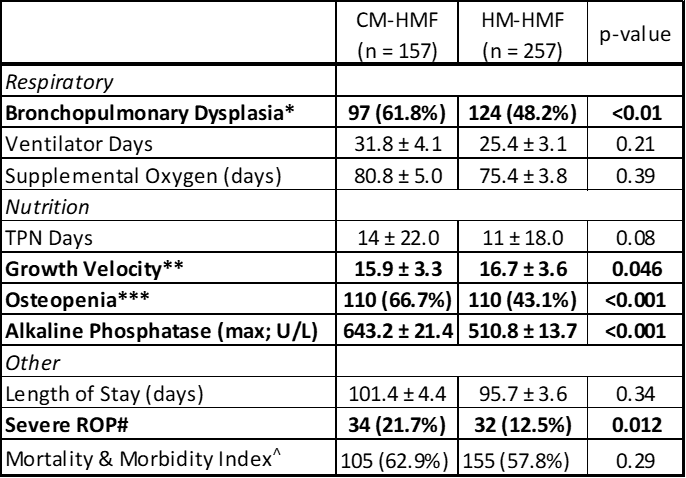
Table 3 provides non-infectious outcomes for infants who survived to discharge or 36 weeks. Infants fortified with a HM-HMF showed statistical reductions in BPD (use of supplemental oxygen or positive pressure at 36 weeks’ CGA), improved growth velocity from day 10 to 36 weeks’ CGA and reduction in severe ROP (stage 3 or greater or the use of Avastin/laser therapy). Additionally, infants fed HM-HMF showed reductions in osteopenia as well as maximal alkaline phosphatase values.
Table 3: Non-infectious outcomes by cohort (survivors to discharge or 36 weeks). Mean +/- SD or N (%)
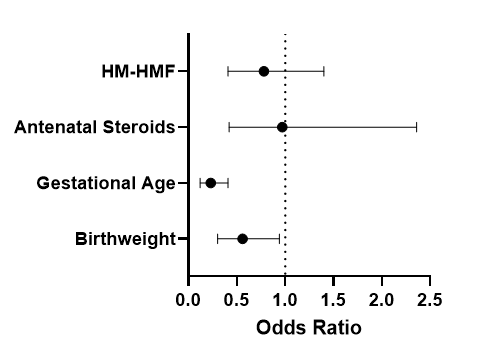
To assess if HM-HMF had an independent effect on patient outcomes, multivariate regression analysis was performed. As gestational age and SGA status were statistically significantly different between cohorts, we utilized scaled (by standard deviation) birthweight and gestational age as variables, as well as the receipt of antenatal steroids and HM-HMF use. In multivariate regression analysis, HM-HMF remained an independent predictor in reducing surgical NEC (OR 0.09, 95% CI 0.005-0.53) as well as BPD (OR 0.23, 95% CI 0.14-0.37).
Table 4 A-C: Multivariate logistic regression of surgical NEC (A), BPD (B), and severe ROP (C) controlled for birthweight, gestational age, antenatal steroid use, and use of HM-HMF.
A) Surgical NEC
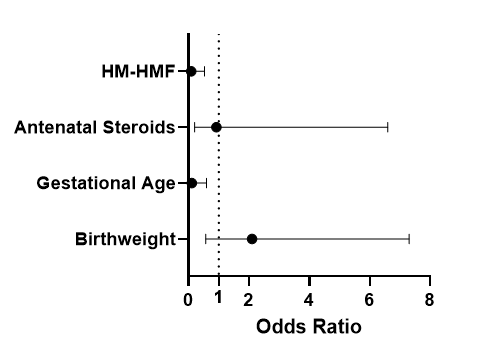
B) BPD
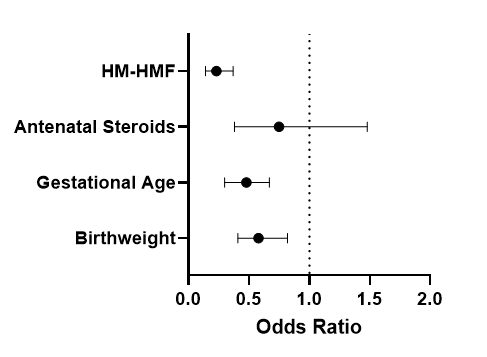
C) Severe ROP
Conclusions:
An exclusive human milk diet utilizing HM-HMF, compared to a human milk diet fortified with liquid CM-HMF, can reduce significant morbidities in an extremely premature infant population. These reductions in morbidities were achieved while maintaining similar or improved growth compared to the CM-HMF cohort. These data add to the growing literature that supports the use of an exclusive human milk diet.
