Protocol Review Committee Members & Procedures
All pharmaceutical, cooperative group, and investigator-initiated protocols either involving patients with cancer or cancer-relevant studies (e.g.: studies about cancer risk factors, prevention, cancer treatment, survivorship or studies that include participants currently or previously diagnosed with cancer or their caregivers) must receive a review by the PRC.
- PRC Submissions should be completed via the OnCore system. If you are unfamiliar with OnCore, you may complete this REDCap form.
- Protocols will be managed depending on level of review
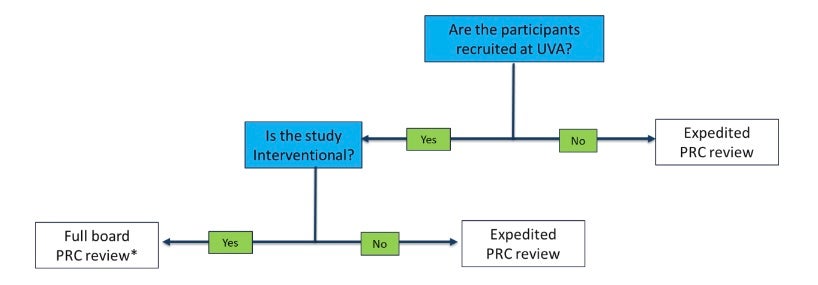
* Interventional studies that are funded through a peer-reviewed mechanism or approved by another NCI-CC PRC are expedited
- PRC Chair, with the assistance of the PRC coordinator, determines if protocol requires full committee review, if it’s eligible for administrative/expedited review, or if it requires no review (exempt) by the PRC.
- Single patient INDs are exempt from PRC review.
- The PRC meets on the second Thursday and fourth Monday of every month, and the deadline for submission is noon two weeks prior to the meeting
- The PRC provides the outcome of review by a letter e-mailed to the investigator
- Submission to the Institutional Review Board for Health Sciences Research will require a final approval letter from the PRC Chair.
If you have questions, please contact Saba Mahmood at sm4pp@uvahealth.org.
PRC Members
Ryan Gentzler, MD
Hematology Oncology
PRC Chair, Department of Medicine
Emily Ayers, MD
Hematology Oncology
PRC Co-chair, Department of Medicine
Roger Abounader, MD
Microbiology, Immunology & Cancer Biology and Neurology
Michael Devitt, MD
Hematology Oncology
Patrick Dillon, MD
Hematology Oncology
Firas El Chaer, MD
Hematology Oncology
Michael Keng, MD
Hematology Oncology
Paul Vincent Viscuse, MD
Hematology Oncology
Christopher McLaughlin, MD
Radiation Oncology
Kari Ring, MD
Gynecologic Oncology
Kara Romano, MD
Radiation Oncology
David Shonka, MD
Otolaryngology
Shayna Showalter, MD
Surgical Oncology
Phil Chow, PhD
Center for Behavioral Health & Technology
Soutik Ghosal, PhD
Department of Public Health Sciences, Division of Biostatistics
Debamita Kundu, PhD
Department of Public Health Sciences, Division of Biostatistics
Hong Zhu, PhD
Department of Public Health Sciences, Division of Biostatistics
Michal Ande, CCRC
Clinical Research Coordinator 3
Ashley Donihee
Clinical Research Coordinator 2
Cecilia Flanagan, MHA, CCRC
Clinical Research Coordinator 3
Cara Hanby, BS, CCRP
Protocol Development Specialist
Alexandra Cash, BS, CCRP
Clinical Research Coordinator 2
Alecia Guishard
Pharmacist
Caroline Jones
Pharmacist
Meredith Mort
Pharmacist
Carolina Pham
Pharmacist
Molly Saville
Pharmacist
Halley Vess
Pharmacist
Saba Mahmood
Senior Compliance Analyst
